FY2011 Annual Report
Brain Mechanisms for Behaviour Unit
Professor Gordon Arbuthnott

Abstract
In 2011, the Brain Mechanisms for Behaviour Unit faced staffing changes with a decrease of postdoctoral researchers for several months and an increase again at the end of 2011. The Unit is currently made up of five individuals. The emphasis has shifted slightly as the Unit has expanded to also work on brain slices in addition to cell culture systems. The Unit’s preliminary results on single cells recordings in mixed corticostriatal cultures were published this year. Although the recordings were a success, the results documented in the cultures, a connection from striatal to cortical neurons – that never happens in brain. Dr. Marianela Garcia Munoz perfected a technique that avoids that issue. The initial results utilizing the new method were presented at the International Brain Research Organization (IBRO) conference in Florence, Italy in July 2011. Dr. Violeta Lopez Huerta and Dr. Omar Jaidar joined the Unit in August and December 2011 respectively, resulting in a noticeable increase in productivity. Dr. Takuya Hikima applied a novel method for examining presynaptic processes in cortical terminals and has demonstrated an action of protein kinase A (PKA) on the likelihood of release from individual terminals.
The collaborative work with several scientists in different countries has been fruitful as well. A collaboration with Professor Brian Hyland from the University of Otago, New Zealand, during his sabbatical in OIST, resulted in the publication of a paper which was awarded the Otago School of Medical Sciences (OSMS) Best Paper award for 2011 (Journal of Neuroscience 2011, 31, 6098-107).
Professor William Staines from the University of Ottawa, Canada worked with the Unit for three weeks in the spring. His work resulted in improvements in our immunohistochemistry that made it possible for Mr. Neil Dalphin (undergraduate University of Otago) to get some promising results during his student internship at OIST from November to February.
Teamwork between our Unit, Dr. Stains’ and Dr. Stephan Theiss’ (Heinrich Heine University of Dusseldorf, Germany) resulted in the paper on cortical development published at the end of 2011.
1. Staff
- Dr. Marianela Garcia Munoz, Group Leader
- Dr. Takuya Hikima, Researcher
- Dr. Violeta Gisselle Lopez Huerta, Researcher
- Dr. Omar Pedro Jaidar Benvides, Researcher
- Neil Dalphin, Short-Term Research Assistant (November 14th, 2011 - February 19th, 2012)
- Hiroko Chinone, Research Administrator
2. Collaborations
- Theme: Isolating genetically marked neurons in culture
- Type of collaboration: Joint research
- Researchers:
- Professor William A. Staines, University of Ottawa, Canada
- Professor Anthony Krantis, University of Ottawa, Canada
- Dr. Sarah Schock, University of Ottawa, Canada
- Theme: Immunolocalization of proteins (L-type calcium channels (CaV1.3) and neurotransmitter receptors) in brain at electron microscope resolution.
- Type of collaboration: Joint research
- Researchers:
- Dr. Beulah Leitch, University of Otago, New Zealand
- Olga Shevtsova, M.Sc., University of Otago, New Zealand
3. Activities and Findings
The main goal within the different experiments carried out by the Unit (in slices or cultures; pre- or postsynaptic neuronal activity) is to dissect the pharmacology and physiology of the actions of dopamine on the basal ganglia network activity.
We have built on the success of the cortical and striatal separated cultures and this year we have begun to look at cortical and thalamic neurons cultured in a similar way. We hope to assemble in vitro different parts of the basal ganglia to achieve controlled conditions in which to study the networks that make up these important structures.
3.1 Corticothalamic activity recorded with calcium imaging.
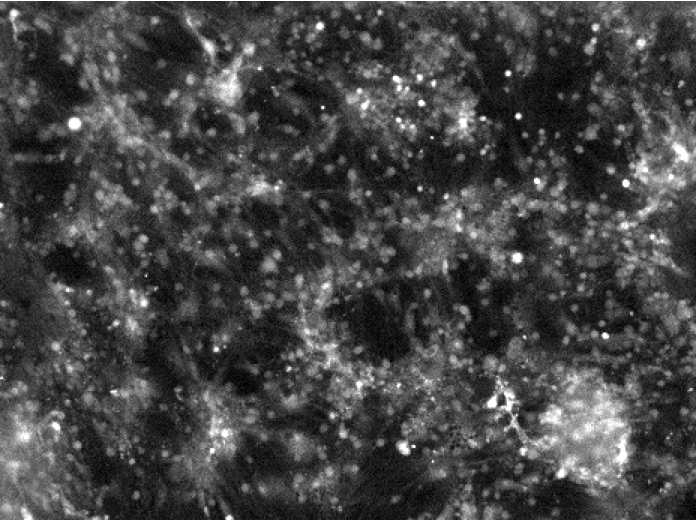
Figure 1.
Cells were loaded with the calcium indicator Fluo5N (Invitrogen) and imaged at the region of the interplay between cortical and thalamic neurons. The dye is brighter in the presence of more calcium. The bright flashes, apparent in the video, represent the consequences of bursts of action potentials allowing increases of intracellular calcium. These flashes are perhaps the cellular equivalent of the bursts seen in these structures in brain during anaesthesia or sleep when the input is reduced. The small white dots (about 15 µm across) are neurons. The time in the video is about 3x real speed.
3.2 Electrical activity in similar paired cultures (as seen in Figure 1) is also spontaneous and synchronous.
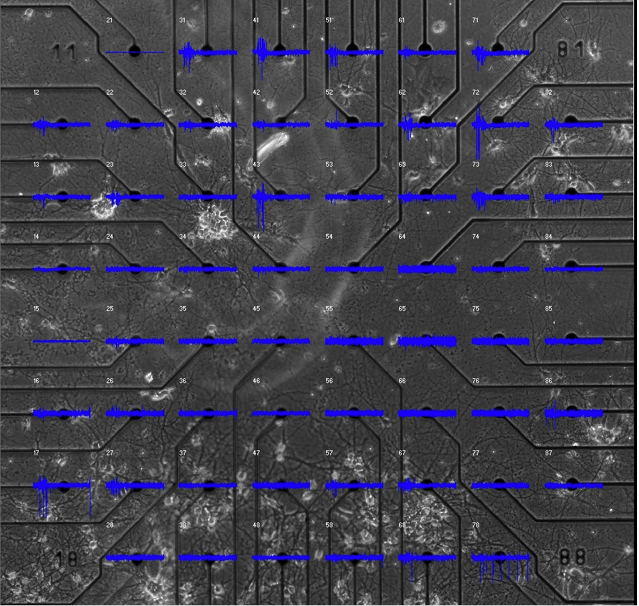
Figure 2.
In this image, thalamic cells are in the lower half of a Multi-Electrode Array (MEA) plate with cortical neurons in the upper electrodes. The microphotograph of the cells plated on the sixty electrodes has been displayed under the electrical recording grid. The electrical activity for 0.5 seconds is displayed above each electrode in blue. The larger vertical lines on the traces are action potentials recorded from neurons in the immediate vicinity of the electrodes. Each electrode records from many cells, however, they can potentially be separated so that the individuals can be distinguished. Even at this increased time resolution it is hard to be sure, where the activity starts.
3.3 Neurons from three different brain regions plated in close proximity
Cryopreserved neurons from E14-16 embryonic mice were plated in three different compartments. Once the neurons settled, the compartments were removed and the cells allowed to develop connections.
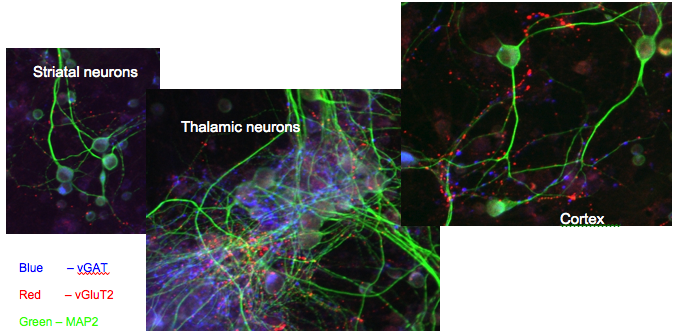
Figure 3.
Photomicrographs from individual areas of a multi compartment co-culture of neurons. The green dye stains microtubule-associated protein (MAP2) present in neuronal dendrites. The blue staining is the vesicular transporter for GABA (vGAT) and marks both inhibitory cells and axon terminals in all three compartments. Also visible in all compartments is the thalamic axon terminal field stained red due to the presence of vGluT2 the vesicular glutamate transporter in thalamic terminals. The presence of the red dots in all three compartments is evidence of innervation of all areas by axons from the thalamic cells.
3.3.1
Dr. Violeta Lopez Huerta has been contributing to the work that Dr. Luis Carrillo Reid did, before his departure, on cortical cultures (Figure 4) and also in some pharmacological experiments on the cortico-striatal system in culture and in slices.
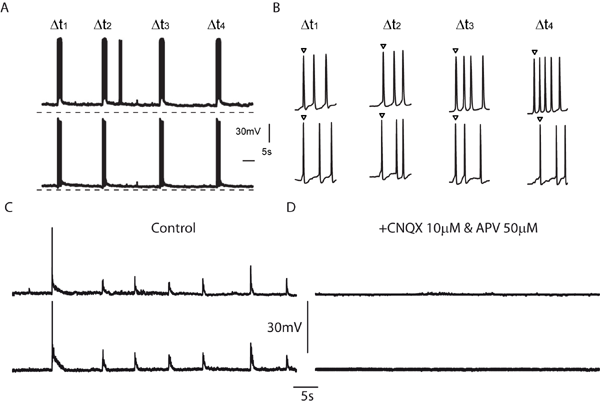
Figure 4.
This figure shows the neuronal synchronization observed in cortical cultures. A. Recordings of spontaneous voltage responses in a pair of neurons reveal that bursts of action potentials occur in a highly synchronized fashion. B. Close up of the initial part of the bursts also illustrates synchronization of single spikes. C. Subthreshold voltage responses indicate that both cells receive the same synaptic input. D. The subthreshold responses can be blocked with glutamate receptor antagonists.
3.3.2
Dr. Omar Jaidar continues to examine the involvement of striatal neurons expressing dopamine receptors D1 and D2 in the mouse model of Parkinsonism (Figure. 5) using calcium-imaging videos in slices of striatum.
Bacterial artificial chromosome (BAC) transgenic mice in which the dopamine D1 receptor expressing neurons were labeled with enhanced green fluorescent protein (EGFP) were injected with 6-OHDA (6-hydroxydopamine) in the substantia nigra. The image demonstrates the typical asymmetric posture following destruction of dopamine cells. Animals challenged with the dopamine receptor agonist apomorphine (0.25 mg/kg, sc) display turning behavior.
3.4 Presynaptic control of neurotransmitter release.
Evidence from previous work from ourselves and others suggests that the major action of dopamine is upon corticostriatal synapses, however the action could be presynaptic as well as postsynaptic. Methods to study presynaptic release on these small terminals in brain are few and challenging. Previously, Dr. Takuya Hikima studied presynaptic release of the granule cells terminal field in CA3 (mossy fibres) using the fluorescent marker synaptopHluorin expressed in transgenic mice. The hippocampal anatomical arrangement of these fibres probably contributed to the large signal observed at that time. Working with corticostriatal cells, Dr. Hikima discovered that this system has a low signal-to-noise ratio. He needed to induce many action potentials in the terminals to be able to see release. However, such long trains of action potentials would themselves change synaptic efficacy. He therefore has applied a new method: he generated a plasmid encoding synaptophysin and the pH-sensitive green fluorescent protein (synpHlourin) and cloned that into a lentiviral vector to infect cortical cells in culture. Using this preparation, he has now been able to observe fluorescent signals with only 5 action potentials, and to detect the known actions of protein kinase A (PKA) on the vesicular dynamics. He is now exploring ways with which he can transfect cortical cells in vivo so that we can use his new probe in brain slices.
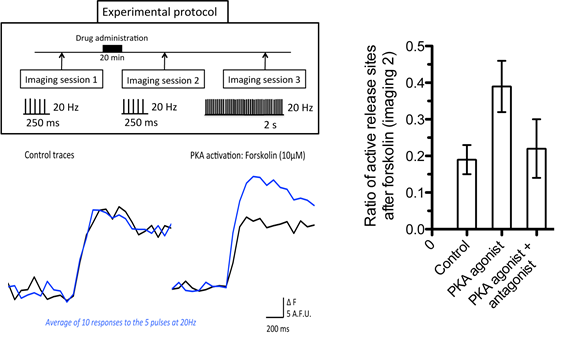
Figure 5. Changes in fluorescence (ΔF) of evoked exocytosis in cortical neurons in culture. As illustrated in the experimental protocol, between imaging sessions 1 and 2 the protein kinase A (PKA) agonist (forskolin 10µM) or control solution was applied in the bath for 20 minutes. The traces are the average response to a set of 5 pulses at 20Hz for 250 ms delivered ten times at 0.2 Hz. Black traces represent imaging session 1 and blue traces illustrate the average response in imaging session 2. Imaging session 3 stimulates the preparation with 40 pulses (20Hz/2s) to obtain an estimate of all possible release sites (not shown). Only areas with more than twice baseline fluorescence were considered as actively releasing. The histogram indicates the ratio of the changes in fluorescence relative to imaging session 2 (i.e., ΔF of imaging session 2/ ΔF of all areas >2x baseline fluorescence). Activation of PKA signal transduction cascade with forskolin (10µM) resulted in an increase in release and an increase in active release sites relative to controls. The PKA antagonist Kt5720 (200nM) blocked the effect of forskolin. The error bars represent the SEM of measurements for 4 different cultures.
3.5 Otago University Joint Research Projects
The final publication from Dr Cyril Dejean’s time in New Zealand suggests that the rhythms seen in the basal ganglia slow wave electrical activity are embedded in a much slower oscillation that influences both the phase and amplitude of oscillations in globus pallidus and striatum. The origin of this activity is not known and has not been detected before. Frequency and phase of the oscillations can be influenced by dopamine receptor activation.
With Dr Beulah Leitch we continue to collaborate on the electron microscopic localization of proteins in the brain. Our most recent study in the ‘stargazer’ mouse suggests that the absence epilepsy that these animals display may be a consequence of the blockade of glutamate GluR4 receptors specifically on the corticothalamic synapses in the reticular nucleus of thalamus. This nucleus is the major source of inhibition in thalamus and therefore the decrease in its activation might lead to hyper-responsiveness in thalamo-cortical networks. The thalamic hyper-responsiveness could lead to the epileptic absence periods characteristic of this type of mutant mice. A manuscript with a description of these results is in press in Neuroscience.
3.6 Ottawa University Joint Research Project
This continuing long-term collaboration has produced very interesting neurohistochemical results. Dr Sarah Schock and Prof Staines are on the author list of two out of three of our publications on the subject and we have another in preparation. The thalamic cells that we have begun to culture (Figures 1-3) were dissected by them and frozen by QBM CellScience (Prof. Anthony Krantis, Chief Executive Officer) and shipped to Okinawa from Canada. We prepared more of those cells as well as the more usual cortex and striatal cells while I visited the University in March. The collaboration with Ottowa University also includes some future challenges such as solving the problems of transfecting cryopreserved neurons. We also continue to work with the “Global Network for Brain Reconstruction”.
3.7 Future plans
The Unit’s 2012-2013 objectives/goals include:
- To continue with the successful work of the Unit on neuronal cultures and brain slices containing areas of the basal ganglia. Several pharmacological projects will potentially explain why slices of cortex and striatum are silent, and how activation of NMDA receptors could affect that.
- To continue exploring in corticostriatal slices the effects of reducing excitatory input onto striatal fast-spiking interneurons as a way to investigate the ideas – generated in hippocampus – about control of activity by “hub” neurons.
- To commence work with intracellular recording in vivo to study the electrophysiology of the thalamocortical circuit that is the output from the basal ganglia. Optogenetic studies on this circuit are part of a collaboration with Professor Bernd Kuhn in OIST (see below).
- To continue our current fruitful international collaborations.
- To develop new collaborations with the:
・Physics and Biology Unit at OIST under the leadership of Professor Jonathan Miller to examine more efficiently the eletrical activity in the MEAs.
・Optical Neuroimaging Unit at OIST under the leadership of Professor Bernd Kuhn to examine, in awake mice, the output pathway from thalamus to cortex that is the final output pathway from the basal ganglia.
・Frey Initiative Research Unit at the Quantitative Biology Center in Riken Kobe under the leadership of Dr. Urs Frey to explore the use of his complementary-metal-oxide-semiconductor (CMOS) integrated circuit for amplification, addressing and analog-to digital conversion of signals from neurons that connect together on multi array electrodes.
4. Publications
4.1 Journals
Dejean C, Arbuthnott G, Wickens JR, Le Moine C, Boraud T, Hyland BI (2011) Power Fluctuations in Beta and Gamma Frequencies in Rat Globus Pallidus: Association with Specific Phases of Slow Oscillations and Differential Modulation by Dopamine D1 and D2 Receptors. The Journal of Neuroscience 31:6098-6107.
Randall FE, Garcia-Munoz M, Vickers C, Schock SC, Staines WA, Arbuthnott GW (2011) The corticostriatal system in dissociated cell culture. Frontiers in Systems Neuroscience 5.
Schock SC, Jolin-Dahel KS, Schock PC, Theiss S, Arbuthnott GW, Garcia-Munoz M, Staines WA (2012) Development of dissociated cryopreserved rat cortical neurons in vitro. Journal of Neuroscience Methods 205:324-333.
4.2 Books and Other One-Time Publications
Arbuthnott GW (2011) Of rats and patients. Some thoughts about why rats turn in circles and Parkinson’s disease patients cannot move normally. In: Contemporary Animal Models of Movement Disorders (Dunett SB, Lane EL, eds), pp 317-323. London: Springer / Humana.
4.3 Oral and Poster Presentations
Nov. 22 Presentation to Dr Deguchi of Shionogi
Studying individual connections between neurons in culture.
Feb. 29 Faculty Lunchtime presentation
Facebook for neurons? Helping isolated neurons form a social network.
March 19 Talk to University of Ottawa
Facebook for neurons? Helping isolated neurons form a social network.
4.4 Posters
Vickers Catherine, Wickens Jeff, Arbuthnott Gordon. (2011) Corticostriatal plasticity and network dynamics in anatomically defined primary neuronal culture system. In: 8th IBRO World Congress of Neuroscience pA 154. Florence, Italy.
Garcia-Munoz M, Carrillo-Reid L, Staines WA, Arbuthnott GW. (2011) NMDA receptor activity in synaptically connected neurons from dissociated striatal and cortical tissue grown on multielectrode arrays. In: 8th IBRO World Congress of Neuroscience pA 112. Florence, Italy.
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
Nothing to report
7. Other
Nothing to report.