FY2019 Annual Report
Cellular and Molecular Synaptic Function Unit
Professor Tomoyuki Takahashi

Abstract
In the presynaptic terminal, neurotransmitters are released from synaptic vesicles (SVs) via exocytosis. After exocytosis, SV membranes are retrieved by endocytosis and SVs are refilled with neurotransmitter to be reused for the next round of neurotransmission. This vesicular recycling/reuse mechanism is crucial for maintenance of synaptic transmission underlying a variety of brain functions. Although mechanisms for SV endocytosis have been well clarified, much less is known for the mechanism underlying SV transport following endocytosis. To study synaptic vesicle transport, dynamics and involvement in brain health and disease, we use several different model systems. We developed a giant synapse preparation in culture (Dimitrov et al, 2016 J Neurosci) that has enabled imaging studies with high signal-to-noise ratio (Guillaud et al, 2017 eLife). Using this preparation, we have further developed a synaptic model of neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease (Dimitrov et al, in preparation). In parallel, we have developed another synaptic model for neuropsychiatric diseases using neurons differentiated from human iPSCs from patients and healthy individuals (Dimitrov et al, in preparation).
SVs are associated with many types of proteins that reside with SVs or transiently interacts with SVs. By elaborating proteomics workflow, we identified 1500 proteins in highly purified SV fraction from rat whole brain, quantified their number per SV and classified them into SV-resident and SV-visitor proteins (Tauofiq et al, paper submitted). This inventory provides us with a resource for studying molecular mechanisms associated with SV dynamics and functions. By combining this SV proteomics with human iPSC-derived neurons, we can compare SV protein profiles between patients with familiar background and healthy siblings. We can also apply imaging and electrophysiological analyses to discover sub-synaptic targets responsible for functional impairments.
Using high-resolution confocal and STED imaging and presynaptic patch-clamp analyses in combination at the calyx of Held, we have demonstrated the presence of microtubules (MTs) in presynaptic terminals in the proximity to ~50% of SVs. Mild depolymerization of MTs slowed the SV recycling time, suggesting that MTs are involved in SV transport (Piriya Ananda Babu, Wang et al, 2020, J Neurosci).
Using patch-clamp analyses, we identified synaptic mechanisms of volatile anesthetics isoflurane (Iso). Iso inhibited release of excitatory transmitter glutamate by blocking voltage-gated calcium channels in presynaptic terminals and SV fusion machinery downstream of calcium influx. During repetitive transmission, Iso impaired action potential transmission from pre- to postsynaptic elements in a frequency-dependent manner, preferentially blocking high-frequency transmission. Such a low-pass filter effect of Iso was also observed in vivo in mouse cerebral cortical synapses. Our findings are consistent with the known fast wave disappearance in EEG during consciousness decline (Wang et al, 2020 J Neurosci).
1. Staff
- Dr. Laurent Guillaud, Group Leader (until December, 2019)
- Dr. Zacharie Taoufiq, Staff Scientist
- Dr. Satyajit Mahapatra, Staff Scientist
- Dr. Han-Ying Wang, Postdoctoral Scholar
- Dr. Soumyajit Dutta, Postdoctoral Scholar
- Dr. Dimitar Dimitrov, Specialist, Technical Staff
- Ms. Anna Garanzini, Technical Staff
- Mr. Francois Beauchain, Technical Staff (until June, 2019)
- Mr. Kojiro Suda, OIST Ph.D. Student lab rotation (from April to August, 2019)
- Ms. Sruthi Raja, Special Student (from May to November, 2019)
- Ms. Tuovinen, Sarianna,OIST Ph.D. Student lab rotation (from September to December, 2019)
- Mr. Alexander David Becalick, Research Intern (from February, 2020 -)
- Ms. Sayori Gordon, Research Unit Administrator
2. Collaborations
2.1 Research theme: Functional analysis of tau protein toxicity
- Name of partner organization: Doshisha University Faculty of Life and Medical Sciences
- Type of collaboration: Joint research
- Principal researcher: Dr. Tetsuya Hori, Doshisha University
- Researcher: Dr. Tomohiro Miyasaka, Doshisha University
2.2 Research theme: Functional and proteomics analyses of psychiatric diseases at patient’s iPSC-derived synapses
- Name of partner organization: University of the Ryukyus
- Type of collaboration: Scientific Collaboration
- Principal researcher: Dr. Masayuki Matsushita, University of the Ryukyus
- Researcher: Dr. Gakuya Takamatsu, University of the Ryukyus
2.3 Research theme: Screening assay of MT-dynamin binding peptides rescuing synapses from tau toxicity
- Name of partner organization: Okayama University
- Type of collaboration: Scientific Collaboration
- Principal researcher: Dr. Kohji Takei, Okayama University
- Researcher: Dr. Tetsuya Takeda, Okayama University
2.4 Research theme: Mechanism of general anesthesia in vivo
- Name of partner organization: Nagoya University
- Type of collaboration: Scientific Collaboration
- Principal researcher: Dr. Takayuki Yamashita, Nagoya University
- Principal researcher: Dr. Takayuki Yamashita, Nagoya University
3. Activities and Findings
3.1 Microtubule and actin differentially regulate synaptic vesicle cycling to maintain high frequency neurotransmission (Piriya Ananda Babu and Wang et al, 2020 Jan, J Neurosci).
Cytoskeletal filaments such as microtubules (MTs) and filamentous actin (F-actin) dynamically support cell structure and functions. In central presynaptic terminals, F-actin is expressed along the release edge and reportedly plays diverse functional roles, but whether axonal MTs are present in presynaptic terminals and play any physiological role remain controversial. At the calyx of Held of rats of either sex, confocal and high-resolution microscopy revealed that MTs enter deep into presynaptic terminal swellings and partially co-localize with a subset of synaptic vesicles (SVs). Electrophysiological analysis demonstrated that depolymerization of MTs specifically prolonged the slow recovery time component of EPSCs from short-term depression (STD) induced by a train of high-frequency stimulation, whereas depolymerization of F-actin specifically prolonged the fast recovery component. In simultaneous pre- and postsynaptic action potential recordings, depolymerization of MTs or F-actin significantly impaired the fidelity of high-frequency neurotransmission. We conclude that MTs and F-actin differentially contribute to slow and fast SV replenishment, thereby maintaining high-frequency neurotransmission.
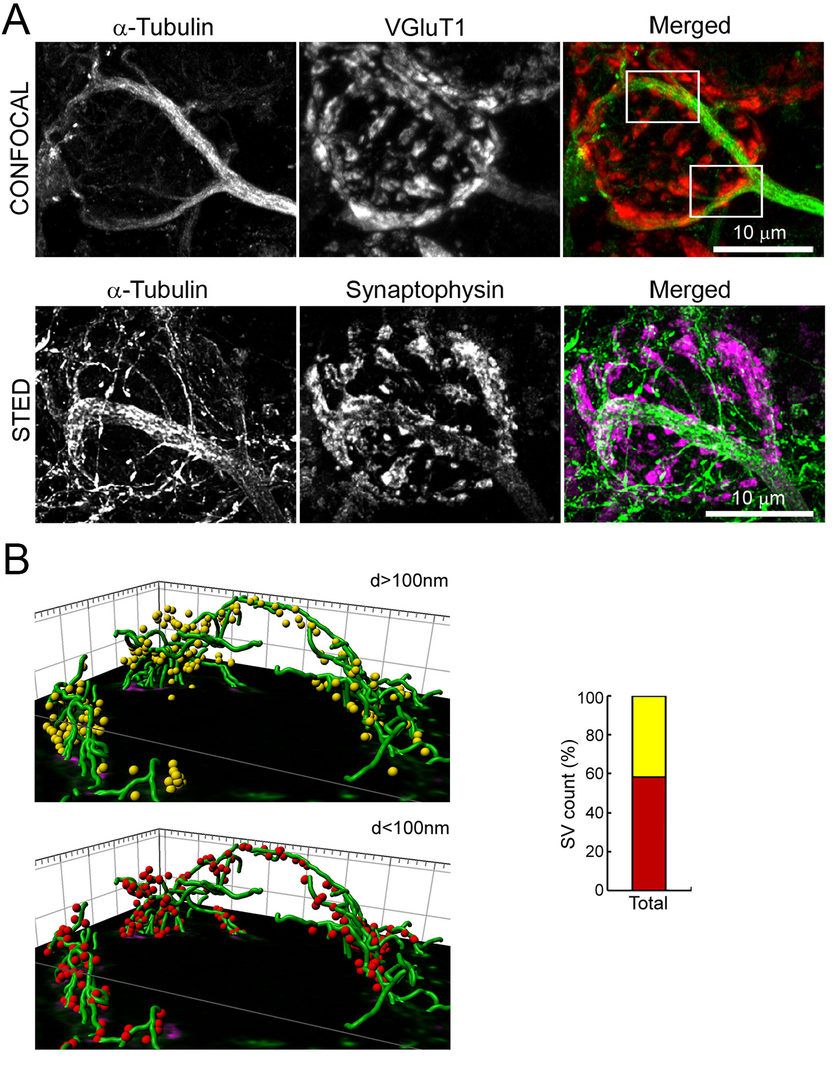
Figure 1 Co-localization of MTs with SVs in a presynaptic terminal at the calyx of Held.
A.Confocal (upper panel) and STED (lower panel) pictures of calyx terminals. MTs (green) overlap with
the SV protein VGluT1 (red) or synaptophysin (magenda).
B.3D reconstruction from STED image. 40% of SVs are distant (>100 nm, yellow) from MTs (green),
but 60% of SVs are closer (<100 nm, red) to MTs.
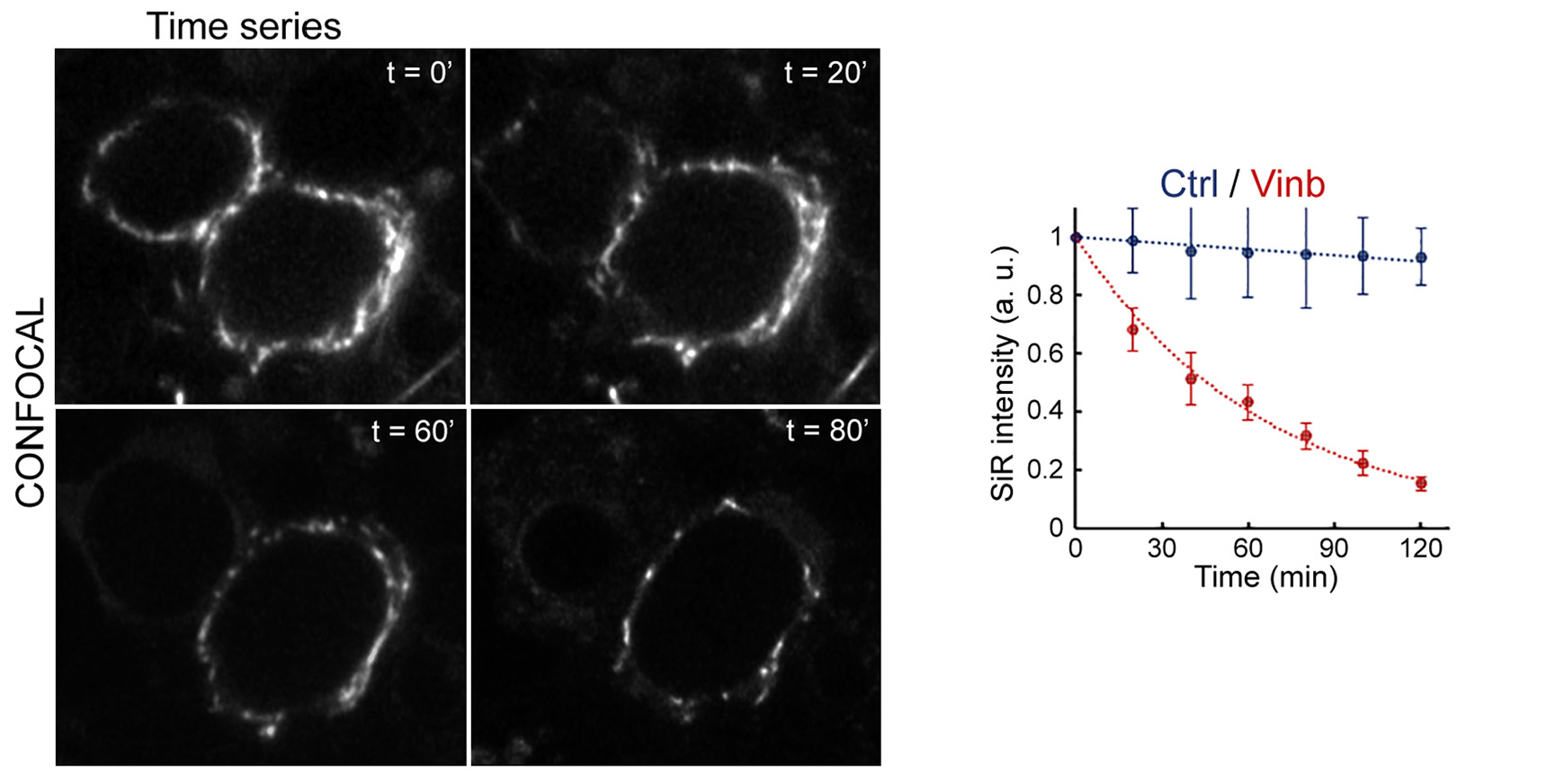
Figure 2 Incubation time-dependent MT depolymerization by vinblastine monitored using SiR-tubulin live-imaging.
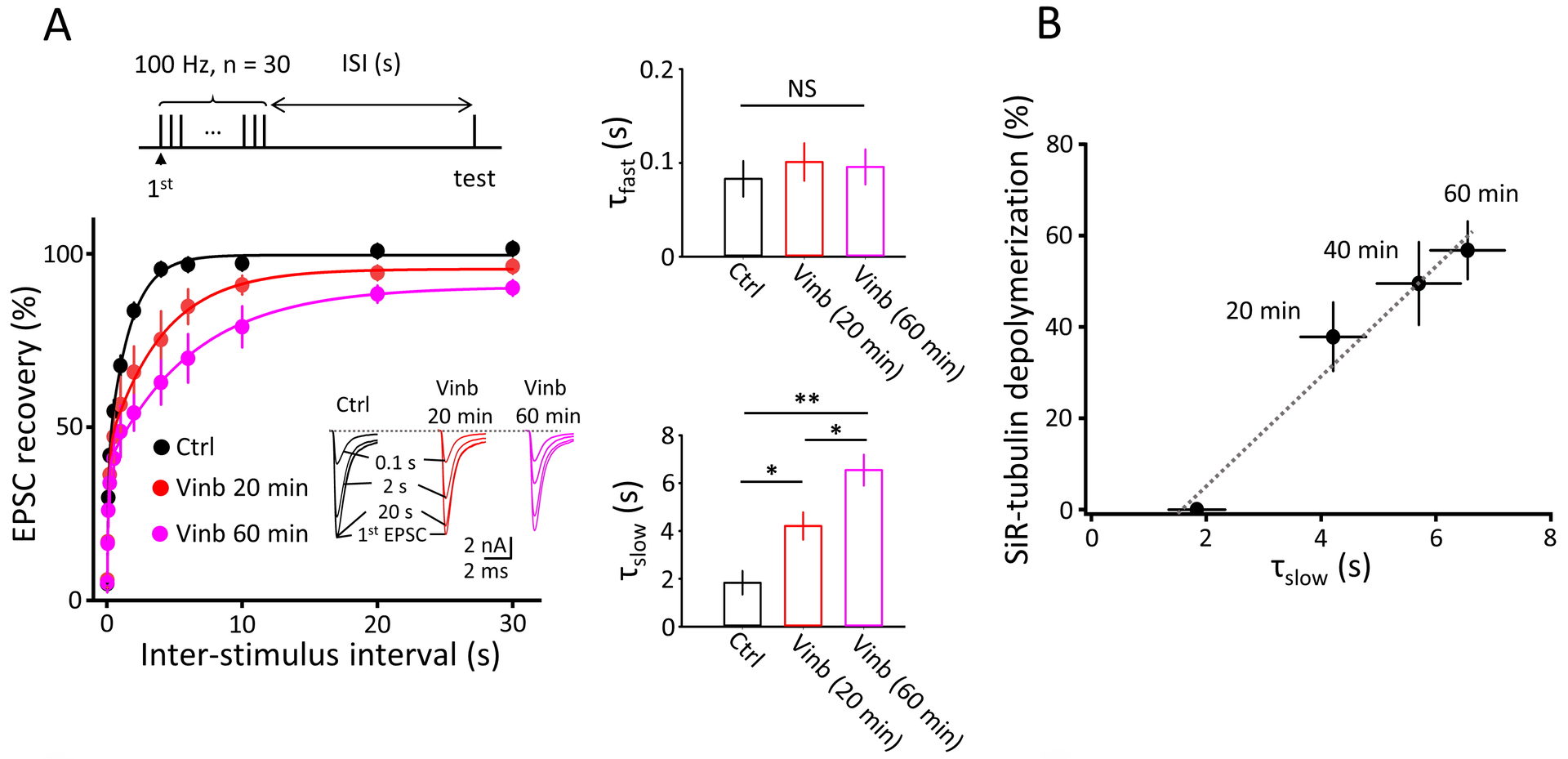
Figure 3 MT depolymerization prolongs the recovery from STD of synaptic response.
A.Prolongation is specific for slow component of bi-exponential recovery (middle bottom bar graphs).
B.Prolongation of slow recovery time constant is proportional to the percentage of MT depolymerization assayed in Fig 2.
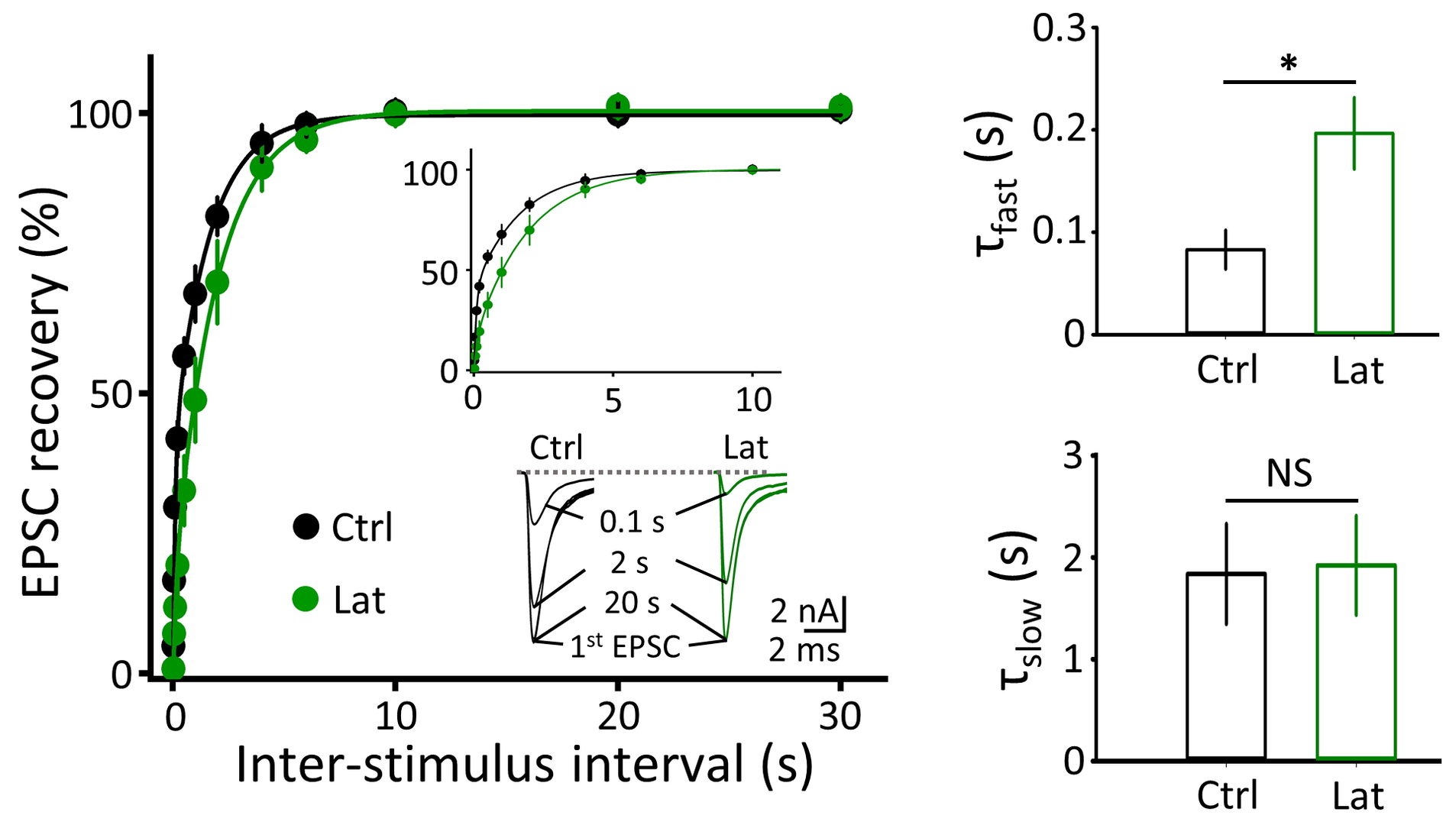
Figure 4 F-actin depolymerization by latrunculin A prolongs the recovery of synaptic response from STD, specifically at its fast component (right top bar graphs)
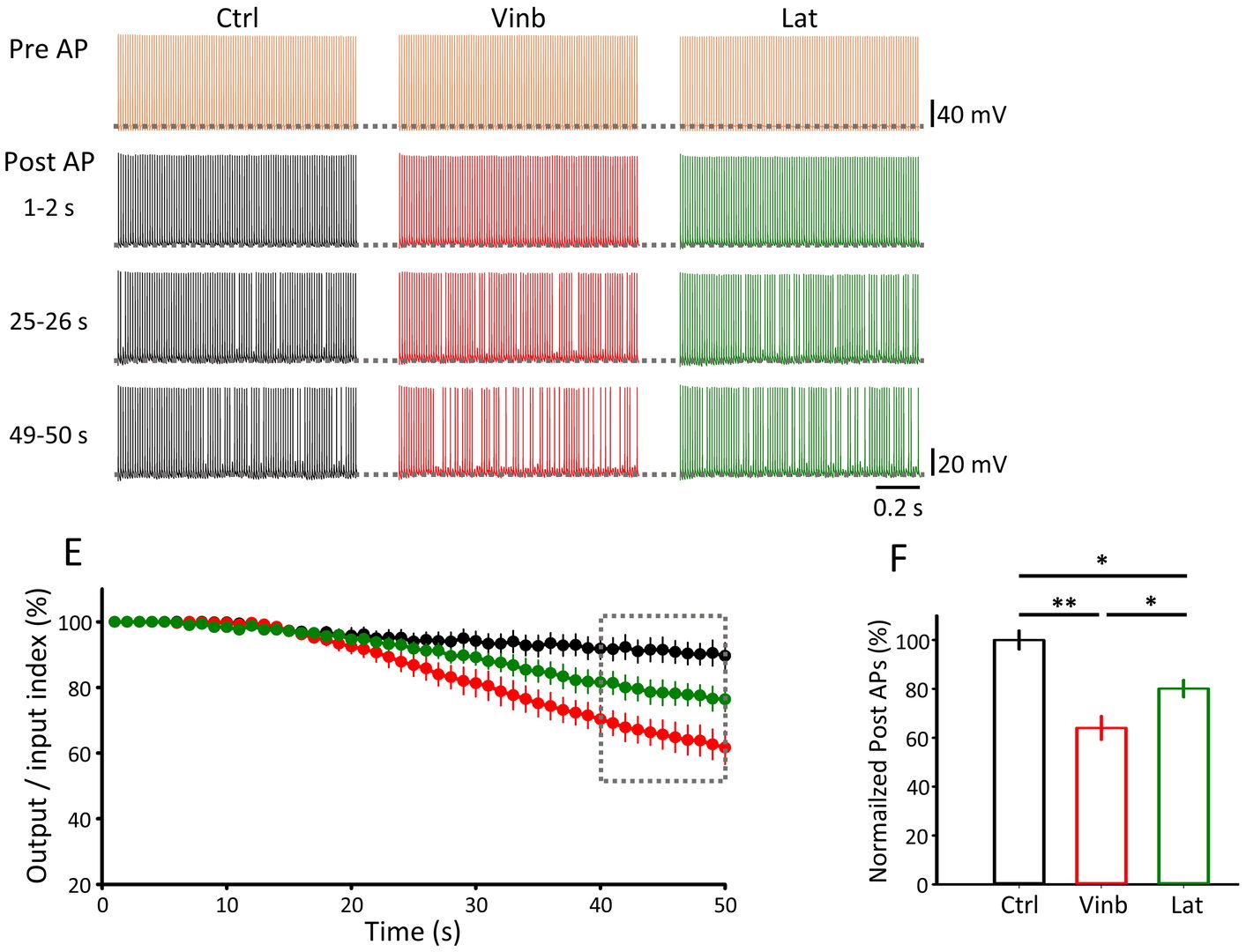
Figure 5 Depolymerization of MT (vinb, red) or F-actin (Lat, green) impairs fidelity of synaptic transmission (postsynaptic AP / presynaptic AP, bottom right bar graphs) measured 40-50 min after depolymerizer treatments.
3.2 Frequency-dependent block of excitatory neurotransmission by isoflurane via dual presynaptic mechanisms (Wang et al, 2020, J Neurosci).
Volatile anesthetics are widely used for surgery, but neuronal mechanisms of anesthesia remain unidentified. At the calyx of Held in brainstem slices from rats of either sex, isoflurane at clinical doses attenuated excitatory post-synaptic currents by decreasing the release probability and the number of readily releasable vesicles.
In presynaptic recordings of Ca2+ currents isoflurane attenuated exocytosis by inhibiting Ca2+ currents evoked by presynaptic depolarization.
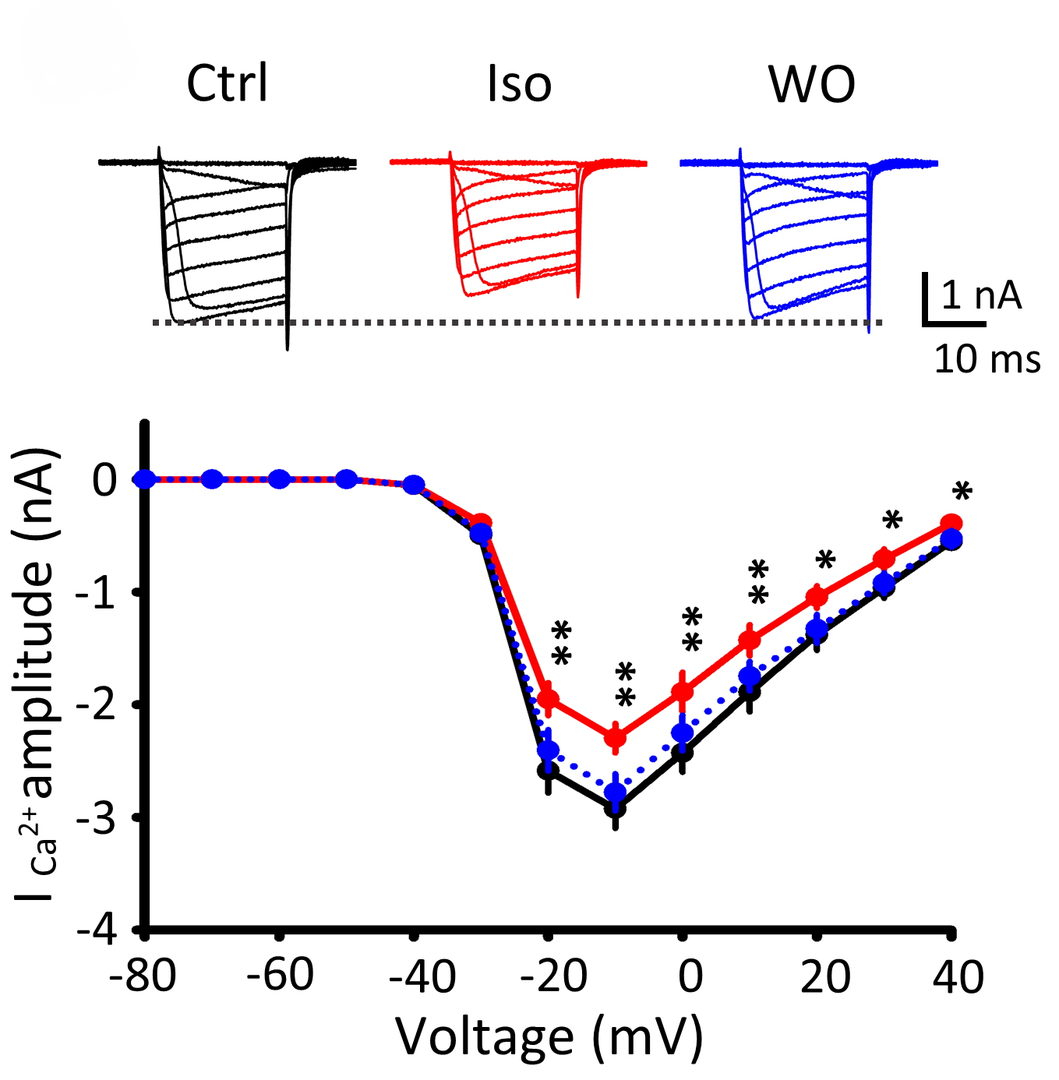
Figure 6 Isoflurane (3%) attenuated presynaptic Ca2+ currents (ICa) evoked by depolarizing pulses (20 ms) of different magnitude at the calyx of Held (records are superimposed). These effects of isoflurane were reversible after washout isoflurane (WO, blue traces). Ca2+ current-voltage relationship indicates significant inhibition of Ca2+ currents by isoflurane.
When the magnitude of Ca2+ currents are gradually increased, vesicle exocytosis initially increases in proportion to Ca2+ currents and reaches a plateau. At this plateau phase, isoflurane still attenuated exocytosis, indicating that it directly inhibits exocytic machinery downstream of Ca2+ influx.
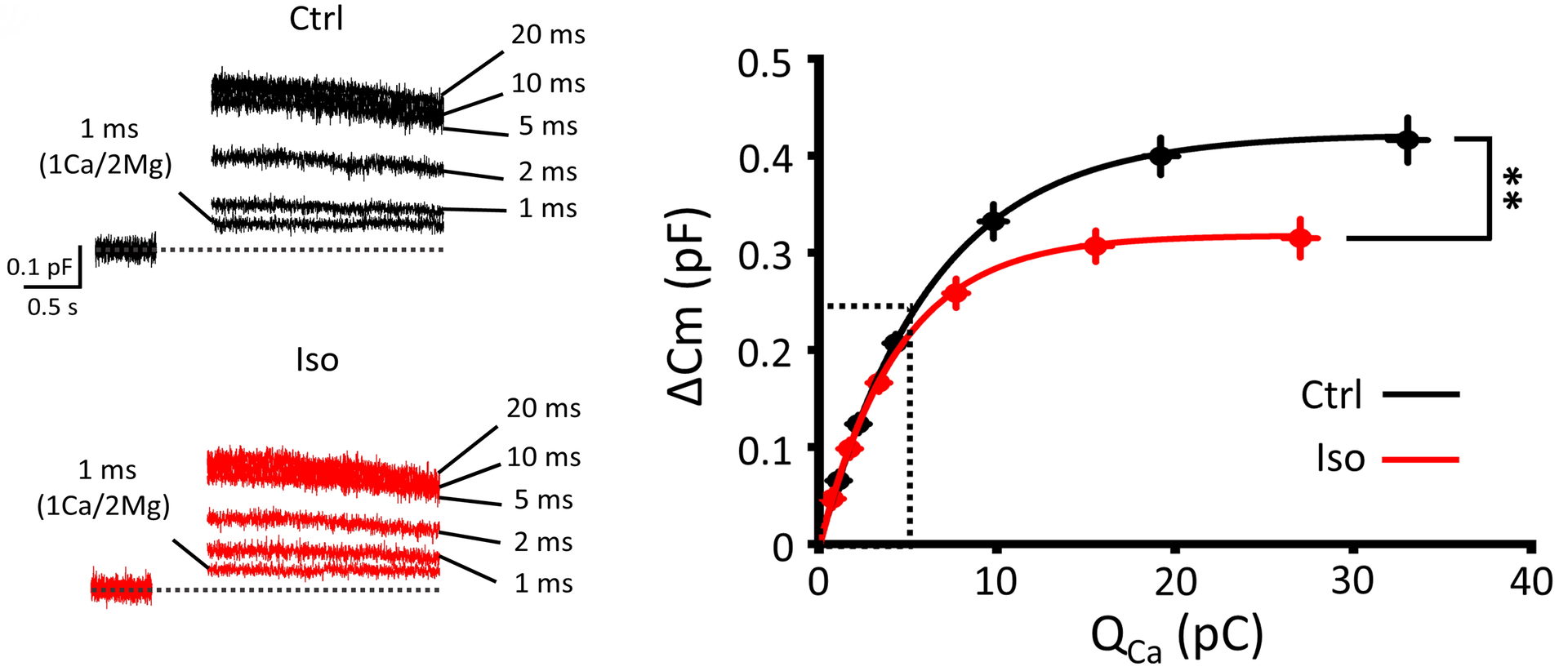
Figure 7 Isoflurane attenuates vesicle exocytosis ΔCm) quantified by capacitance measurements at a plateau phase of Ca2+ current-exocytosis relationship.
Thus, isoflurane exclusively targets voltage-gated Ca2+ channels for a relatively mild exocytosis such as those evoked by a single action potential or at low frequency stimulation, but it additionally targets exocytic vesicle fusion machinery for a massive exocytosis such as those evoked by trains of high frequency stimulation.
We therefore examined the effect of isoflurane on neurotransmission at various frequencies. To this end, we performed simultaneous whole-cell recording of presynaptic and postsynaptic APs. Isoflurane was applied at 1.5 % (1 Mac; clinical dose) or 3 % (2Mac).
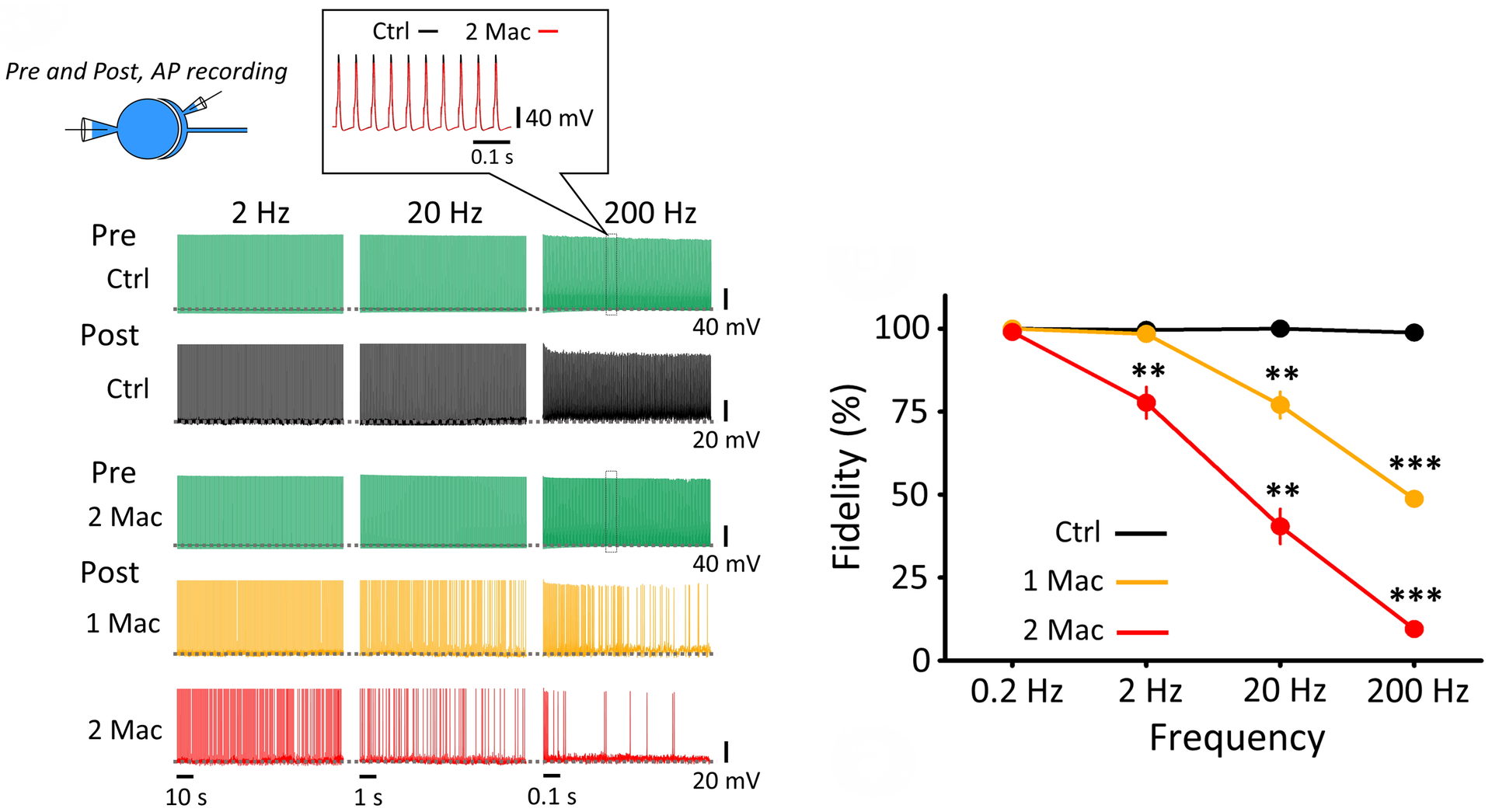
Figure 8 Isoflurane selectively attenuated higher frequency neurotransmission. Pre- and postsynaptic AP recordings at PT. Presynaptic APs (green) do not fail up to 200 Hz in the absence (top raw) or presence (3rd raw) of isoflurane (3%, 2 Mac). Likewise, postsynaptic APs do not fail in control (black). Isoflurane (1.5 %, 1 Mac) had no effect on neurotransmission at or below 2 Hz, but impaired neurotransmission at higher frequencies. Right panel graph summarizes frequency-dependent inhibitory effect of isoflurane.
We next investigated whether such frequency-dependent effect of isoflurane is observed in animals in vivo. To this end, we utilized channel rhodopsin expressing mice and inspired isoflurane (1 Mac). APs were recorded from cerebral sensory cortical neurons in layer 5 using multi-channel extracellular probe and monosynaptic neurotransmission was triggered by optical stimulation of motor neurons. In response to blue light stimulation pulse, sensory neurons generated APs within 30 ms from stimulations. Isoflurane inhalation markedly inhibited AP firings.
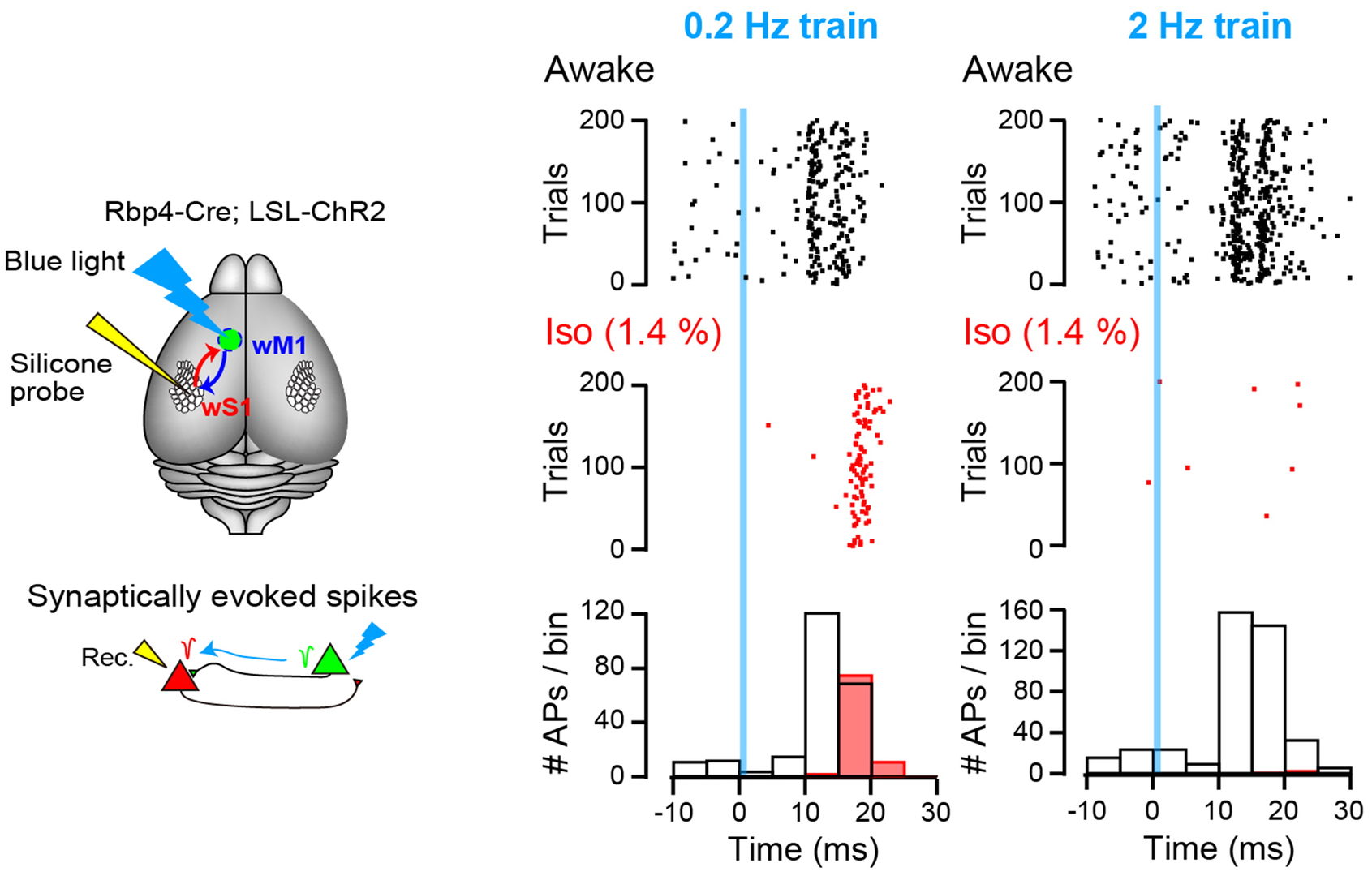
Figure 9 Left schemes indicate experimental set-up. Right plots show AP firings with dots before (awake, black dots) and after isoflurane inhalation (Iso, red dots). Light stimulation pulses were applied at time 0 (blue line) at 0.2 Hz (left) or 2 Hz (right). Bottom histograms indicate number of APs within 5 ms bins.
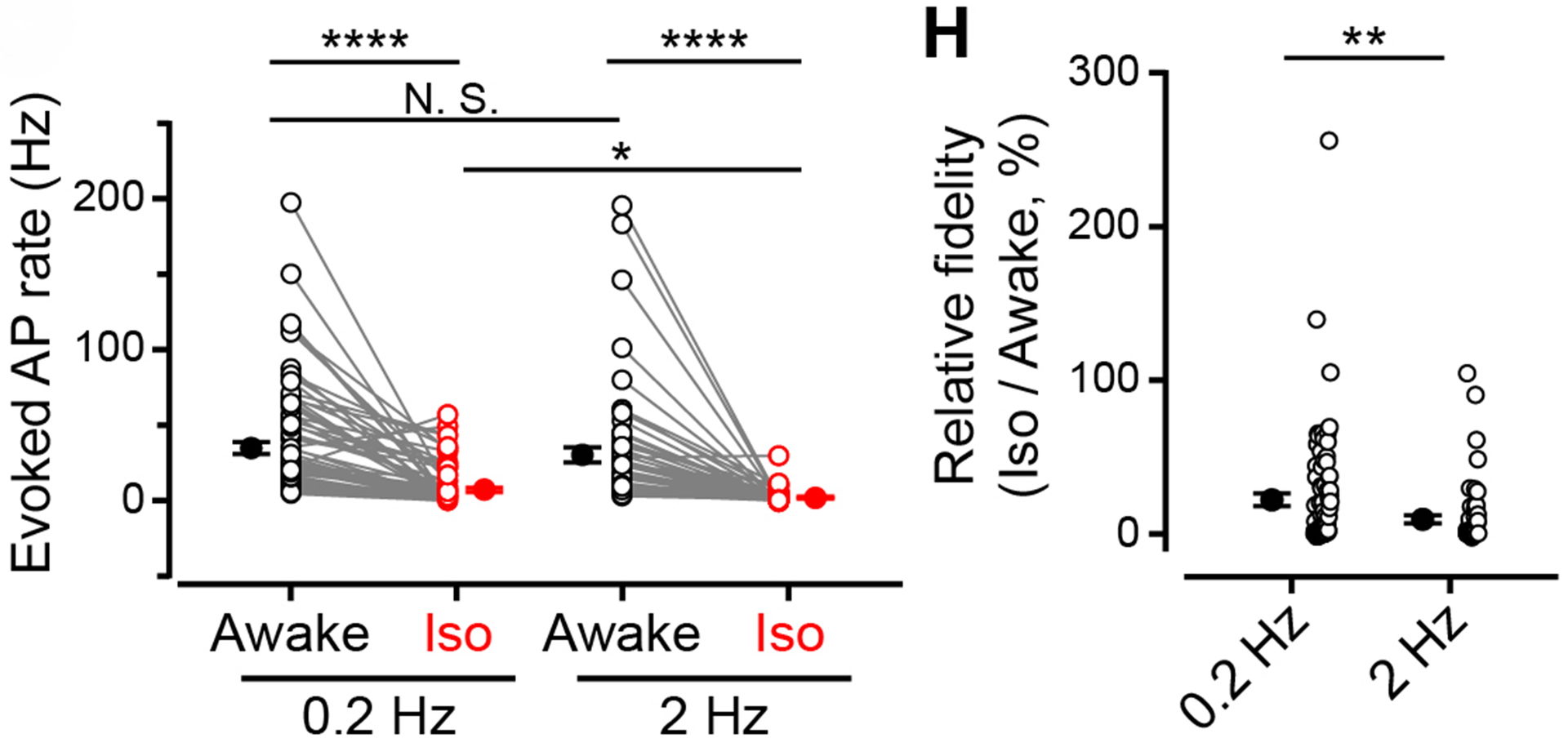
Figure 10 Left panel, number of observed APs per second in response to 0.2 Hz (left) or 2 Hz (right) light stimulation, before (black) and after (red) isoflurane inhalation. Individual and average values are shown. Right panel indicates percentage decrease of AP firing after isoflurane inhalation for neurotransmission evoked at 0.2 Hz or 2 Hz light stimulation.
Thus, dual presynaptic mechanisms operate for the anesthetic action of isoflurane, of which direct inhibition of exocytic machinery plays a low-pass filtering role in spike transmission. Weaker effect of anesthetics on low-frequency transmission seems favorable for the maintenance of life-supporting basal neurotransmission. Low-pass filtering effect of isoflurane is also consistent with large-scale slow-wave synchronization of cortical neurons during anesthesia. Since high-frequency neuronal activity plays essential roles in the maintenance of consciousness, cognition and motor-control, selective inhibition by volatile anesthetics of high-frequency transmission will effectively attenuate such integral neuronal functions, with minimal inhibition of basal neuronal functions.
4. Publications
4.1 Journals (OIST researchers are underlined)
- Piriya Ananda Babu L, Wang H-Y, Eguchi K, Guillaud L, Takahashi T (2020). Microtubule and actin differentially regulate synaptic vesicle cycling to maintain high frequency neurotransmission J Neurosci 40, 131-142.
-
Wang H-Y, Eguchi K, Yamashita Y, Takahashi T (2020). Frequency-dependent block of excitatory neurotransmission by isoflurane via dual presynaptic mechanisms. J Neurosci. 20 May 2020, 40(21):4103-4115; doi:10.1523
- Takahashi T (2019). Presynaptic black box opened by pioneers at Biophysics Department in University College London. Neuroscience, Review article in press.
4.2 Book(s) and other one-time publications
Nothing to report.
4.3 Oral and Poster Presentations
Invited talks
Special lecture “Synaptic function and brain disease”. Tomoyuki Takahashi, in a Conference of Structural Understanding of Multi-scale Psychopathology, Grant-in-Aid for Scientific Research on Innovative Areas. Izuyama, Atami, Japan. 31st August, 2019
“Multi-disciplinary approach to presynaptic black box” Tomoyuki Takahashi, in KAIST-OIST Symposium, OIST main campus. 11th November, 2019
“Multi-disciplinary approach to presynaptic black box” Tomoyuki Takahashi, in the 2nd Osaka University-OIST Symposium, OIST main campus. 20th January, 2020
Oral Presentations
"Deep Synaptic Proteome Dynamics in Mental Health and Disease". Zacharie Taoufiq, in OIST-National Tsing Hua University Joint Seminar: 'Accelerating Research Towards Applications in the Life Sciences'. OIST main campus, 21st August, 2019.
“Uncharted synaptic vesicular proteome”. Zacharie Taoufiq, in Ribbon Synapses Symposium 2019, Max Planck Institute, Göttingen, Germany. September 2nd - 3rd, 2019.
"Functional roles of cytoskeletal proteins in presynaptic terminals". Tomoyuki Takahashi, in the annual synaptic meeting, the National Institute for Physiological Sciences(NIPS), Okazaki, Aichi. 19th- 20th September, 2019.
“Deep synaptic exploration in mental health and disease”. Zacharie Taoufiq, in OIST-Osaka Joint Seminar, Neuroscience-Immunology and cutting-edge technologies, flash talk. OIST main campus.
January 20th-21st, 2019.
Poster Presentations
"Development of induced pluripotent stem cell model of bipolardisorder derived from an Okinawan pedigree with a potentialgenetic component". Gakuya Takamatsu 1, 2, Kumiko Yanagi 6, Yoko Manome 5, Kae Koganebuchi 4, Junsoku Lee 1, Kanako Toyama 1, Mutsumi Isa 3, Kotaro Hattori 7, 8, Tomoko
Hayakawa 9, Chikako Miyauchi Hara 5, Minami Hasegawa 5, Dimitar Dimirov 10,
Tomoyuki Takahashi 10, Hiroshi Kunugi 7, Tsuyoshi Kondo 2, Ryosuke Kimura 3, Tadashi Kaname 6, Hirotaka James Okano 5, Masayuki Matsushita 1. in The 42th Annual Meeting of the Molecular Biology Society of Japan, Fukuoka International Congress Center. December 3-6, 2019.
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
Nothing to report
7. Other
7.1 Teaching class for OIST Ph.D. students ‘Mass Spectrometry based Proteomics', organized by Prof.
Maruyama.
‘LC-MS and analysis of protein modification’ part of this course ‘Mass Spectrometry based Proteomics’
Date: 5th February, 2020
Lecturer: Zacharie Taoufiq



