FY2014 Annual Report
Cellular and Molecular Synaptic Function Unit
Distinguished Professor (Fellow) Tomoyuki Takahashi

Abstract
In presynaptic terminals, synaptic efficacy and precision are determined by the coupling of voltage-gated Ca2+ channels (VGCCs) to vesicles, but the nanoscale topography of VGCCs and their proximity to vesicles remain unknown. To investigate the determinants of VGCC-vesicle coupling, in FY 2014, we combined freeze-fracture replica immunogold labeling of Cav2.1 channels, local [Ca2+] imaging, and patch pipette perfusion of EGTA at the calyx of Held, in collaboration with laboratories in France, UK and Austria. Between postnatal day 7 and 21, VGCCs formed variable sized clusters, vesicular release became less sensitive to EGTA, whereas fixed Ca2+ buffer properties remained constant. Experimentally constrained reaction-diffusion simulations suggest that Ca2+ sensors for vesicular release are located at the perimeter of VGCC clusters. Shortening of the cluster to sensor distance from 30 to 20 nm accounted for developmental changes in EGTA sensitivity and release time course. Thus, we have proposed “perimeter release model”, which provides a unifying framework predicting that the number of VGCCs per cluster determines vesicular release probability without altering synaptic precision. These results were reported in Neuron in January issue of 2015 as Nakamura Y, Harada H, Kamasawa N, Matsui K, Rothman JS, Shigemoto R, Silver AR, DiGregorio, DA, Takahashi T (2015). Nanoscale distribution of presynaptic Ca2+ channels and its impact on vesicular release during development. Neuron, 85, 145-158.
1. Staff
Dr. Tomoyuki Takahashi, Professor
Dr. Hiroshi Takagi, Group Leader, Staff Scientist
Dr. Kohgaku Eguchi, Staff Scientist
Dr. Tetsuya Hori, Staff Scientist
Dr. Laurent Guillaud, Staff Scientist
Dr. Setsuko Nakanishi, Staff Scientist (until December, 2014)
Dr. Zacharie Taoufiq, Staff Scientist
Dr. Masashi Ohmachi, Postdoctoral Scholar
Dr. Dimitar Dimitrov, Specialist, Technician
Ms. Lashmi Piriya Ananda Babu, OIST Ph.D. Student
Ms. Shivani Sathish, Research Intern (from January to July, 2014)
Mr. Abdelmoneim Farid Abdelaziz Elsayed Eshra, Special Research Student (from February to August, 2014)
Ms. Sayori Gordon, Research Administrator
2. Collaborations
- Theme: Regulatory mechanisms for transmitter release
- Type of collaboration: Joint research
- Name of principal researcher: Tomoyuki Takahashi
- Researchers:
- Naoto Saitoh, Doshisha University
- Takafumi Miki, Doshisha University
- Theme: Distribution of presynaptic calcium channels and its impact on vesicular transmitter release during development
- Type of collaboration: Scientific collaboration
- Name of partner organization: Pasteur Institute
- Name of principal researcher: David A, DiGregorio
- Researchers:
- David A, DiGregorio, Pasteur Institute
- Yukihiro Nakamura, Pasteur Institute
- Theme: Distribution of presynaptic calcium channels and its impact on vesicular transmitter release during development
- Type of collaboration: Scientific collaboration
- Name of principal researcher: Ryuichi Shigemoto
- Researchers:
- Ryuichi Shigemoto, the institute of Science and Technology Austria
- Harumi Harada, the institute of Science and Technology Austria
- Theme: Distribution of presynaptic calcium channels and its impact on vesicular transmitter release during development
- Type of collaboration: Scientific collaboration
- Name of principal researcher: R. Angus Silver
- Researchers:
- R. Angus Silver, University College London
- Jason S. Rothman, University College London
3. Activities and Findings
(1) Ca2+ channel-sensor coupling topography and distance at the calyces of Held of developing rodents (Nakamura et al, 2015, Neuron ).
Fast and precise neurotransmission is thought to require tight coupling of Ca2+ channel and Ca2+ sensor on vesicles with a nanometer order coupling distance. Efforts have been made to model the Ca2+ channel-sensor coupling, but the coupling topography remains open because of a lack of information as to the Ca2+ channel distribution and release sites of vesicles in the nerve terminal. The coupling distance between Ca2+ channels and Ca2+ sensors on synaptic vesicle cannot be measured directly, but it is assessed from Ca2+ chelator experiments and classified into two categories; one class is microdomain coupling, where the coupling distance is hundreds of nanometers and slow binding Ca2+ buffer EGTA loaded into terminals blocks transmission as effective as the fast buffer BAPTA. The other category is the nandomain coupling with tens of nanometer coupling distance. In this coupling, EGTA is totally ineffective.
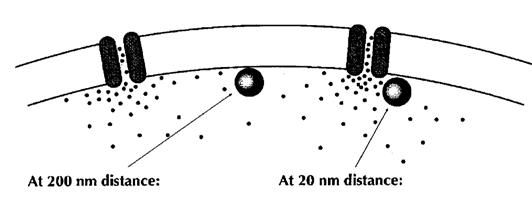
From Neher 1998 Neuron
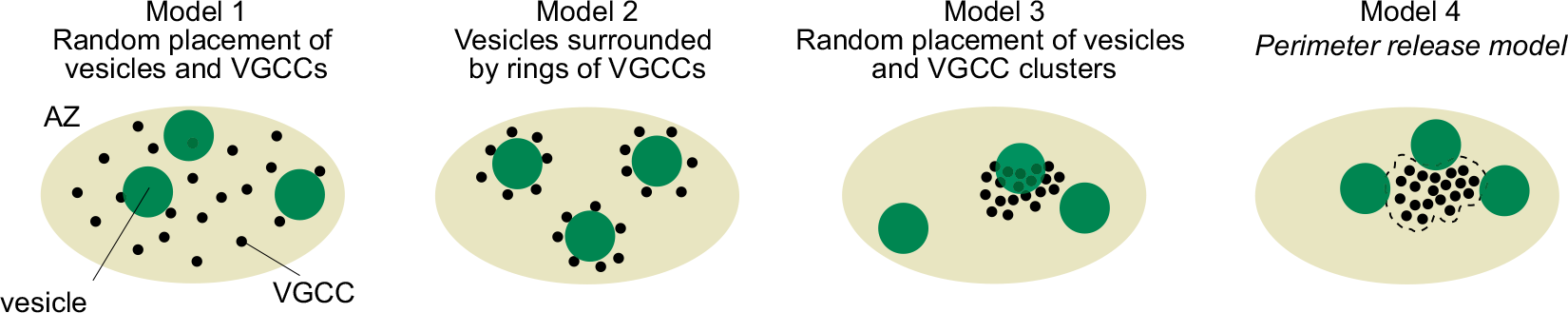
However, there was no information on the localization of Ca2+ channels or their topography relative to docked/primed vesicles in the nerve terminal. The possible topographies include random localizations of channels and vesicles, vesicles surrounded by Ca2+ channels, random placement of vesicle and Ca2+ channel clusters, and vesicles localized near the perimeter of Ca2+ channels.
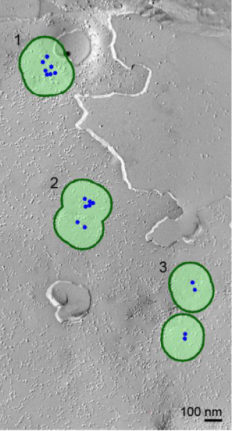
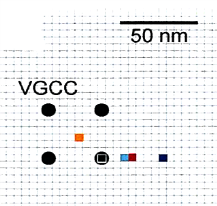
So, we first investigated Ca2+ channel distribution in the calyx terminal using the freeze-fracture replica immunogold labeling of α1A subunit of P/Q type Ca2+ channel, or Cav2.1.
As shown on the right panel, these Ca2+ channels are found 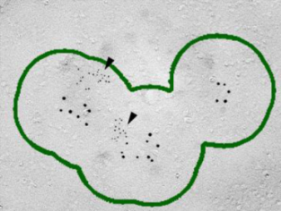 in clusters in the calyx terminal. We also labeled the active zone (AZ) protein RIM1 with a smaller immune- gold particles (arrowheads), which are found close to Ca2+ channels.
in clusters in the calyx terminal. We also labeled the active zone (AZ) protein RIM1 with a smaller immune- gold particles (arrowheads), which are found close to Ca2+ channels.
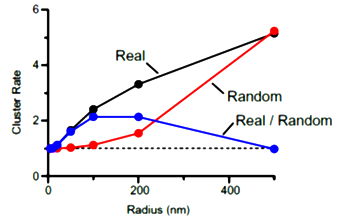 In cluster analysis, we tentatively defined a cluster
In cluster analysis, we tentatively defined a cluster
with a circle of 100 nm in radius as this was the optimal size to distinguish random distribution from cluster distribution. In this criterion, merged clusters are regarded as one cluster.
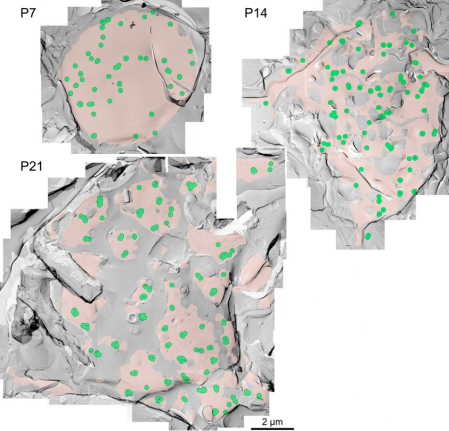
The inter-cluster distance was similar to the distance between AZs previously reported for the calyx of Held. Together with the co-existence of Rim 1, we conclude that these Ca2+ channels are localized as clusters in AZ. This VGCC clustering is consistent throughout development from postnatal day 7 to 21 in rat calyx terminals.
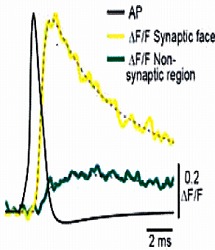
We next loaded ORG-BAPTA (100 μM) into calyx and recorded Ca2+ transients induced by AP from various confocal spots on the terminal identified with co-loaded Alexa dye. In the spot facing the synaptic cleft, but not on the opposite sites, a fast Ca2+ transient was induced by an AP.
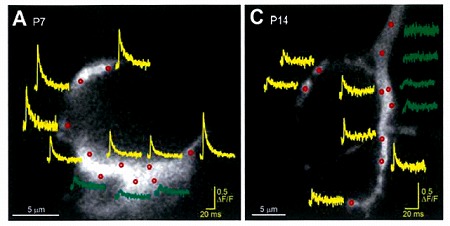
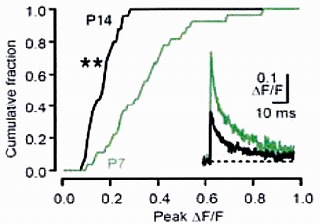
On average, the magnitude of the Ca2+ transient became smaller by 50% from P7 to P14, and this is mainly
because of presynaptic AP becoming shorter during this period.
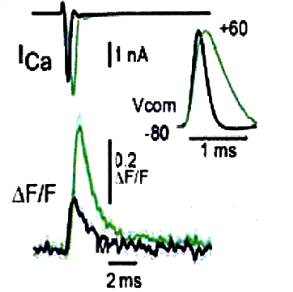
This developmental reduction of Ca2+ transients can be reproduced in P7 calyces by inducing Ca2+ transients with a command pulse in the shorter AP waveform of P14 rats.
During the whole cell recording, mobile 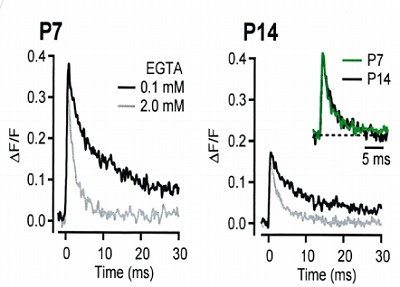
molecules are washed out, but immobile
Ca2+ buffers are still present. In order to
know its properties, we ompared Ca2+
transients with pipette solution containing
0.1 mM EGTA or 2 mM EGTA at P7 and
P14 calyx. As shown on the right panel,
the decay of Ca2+ transients are determined
predominantly by the EGTA concentration,
with little developmental change detectable in
normalized and superimposed traces.
This suggests that endogenous fixed buffer
remains similar in strength and properties
throughout development.
In our simulation, the decay time was best fit by assuming a low 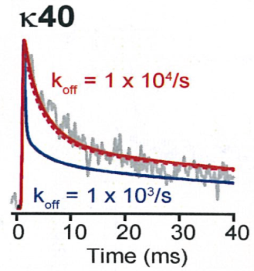
affinity buffer with Kd 100 μM and the Ca2+ binding ratio k = 40,
whereas the simulation on higher affinity buffer or lower k
value deviated from the real time course of Ca2+ transients.
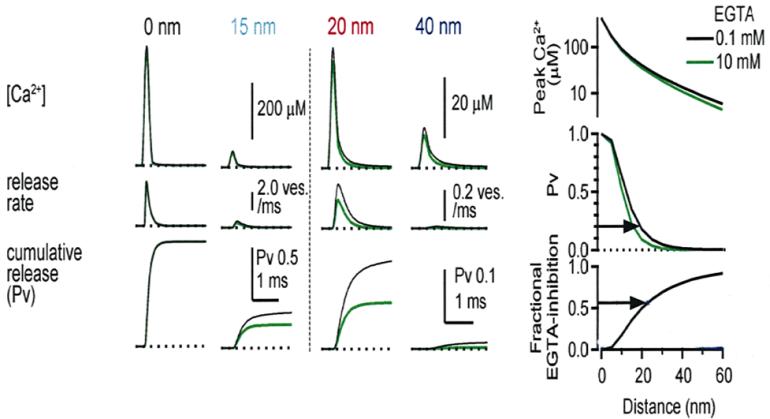
We then tested the effect of EGTA on the synaptic transmission by loading it into the calyx at different postnatal periods. Compared with BAPTA, ![]()
EGTA has a 40 times slower the Ca2+ binding on-rate, therefore it can provide information on the distance between Ca2+ and target. To load high concentration 
EGTA, we used our pipette perfusion techniques through a fine tube installed in a patch pipette.
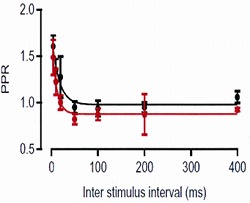
In a control pipette solution we included 0.1 mM EGTA (red trace), as it closely mimicked the endogenous mobile buffer condition (black), with respect to the paired-pulse ratio (PPR: the second
EPSC amplitude relative to the first one) at different inter-pulse intervals.
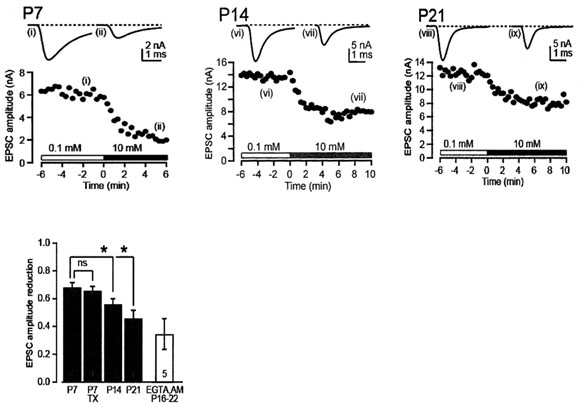
Main results are shown in the next panel. EGTA loaded at 10 mM into calyx terminal, by switching from 0.1 mM EGTA, attenuated the EPSC amplitude by 70% at P7 and by 40-50% at P14 and P21. We also tested membrane permeable EGTA-AM applied from outside to intact calyx at P16-22 and confirmed the persistent EGTA sensitivity throughout this developmental period.
With all these experimental data, we made a simulation.
Because Ca2+ channels are clustered, and 10 mM EGTA
attenuates release by >40%, placement of the Ca2+ sensor is
constrained to outside of the cluster.
In EM studies, the number of Ca2+ channels in a cluster varied a lot, but 20-30 on average throughout development. In this model 4 channel cluster was assumed, and Ca2+ concentrations were estimated at different distances from the perimeter of the cluster.
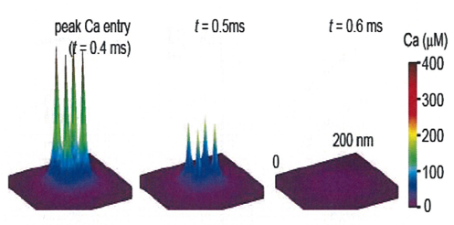
This provided transmitter release rate and release probability at each spot. As shown in the following panel, 50% inhibition of release by EGTA is attained at 20-30 nm away from the cluster.
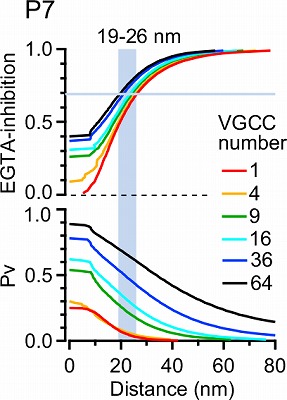
This simulation in the following panel assumes various number of Ca2+ channels in a cluster, ranging from 1 to 64 in different colors for P7 and P14 calyx. The abscissae are the distance from the nearest open channel. At P7, 70% EGTA inhibition, observed in experiments, gives distance of 20-30 nm, whereas at P14, the coupling distance is shorter by 10-20 nm. In any case, the coupling distance is in tens of nanometer scale throughout P7 to P14 and not much affected by the number of channels. In contrast, the release probability at a vesicle highly depends on the channel number, both at P7 and P14.
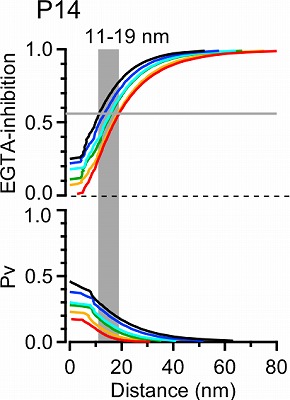
How does this coupling distance affect synaptic precision?
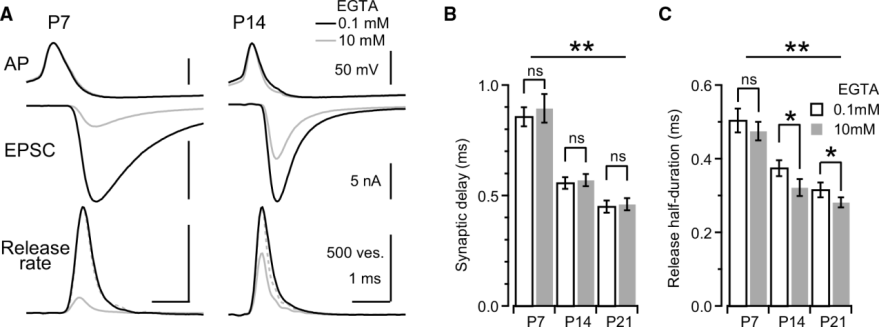
The synaptic precision can be assessed by the synaptic delay from the AP peak to the EPSC onset, and also by the half duration of the release rate that is obtained by deconvolution of EPSCs with miniature EPSCs as the number of exocytic vesicles.
Next panel shows experimental data at P7 and P14 calyces with 0.1 mM or 10 mM EGTA. Both the synaptic delay and release duration become shorter during development and 10 mM EGTA has a minor effect on these precision parameters.
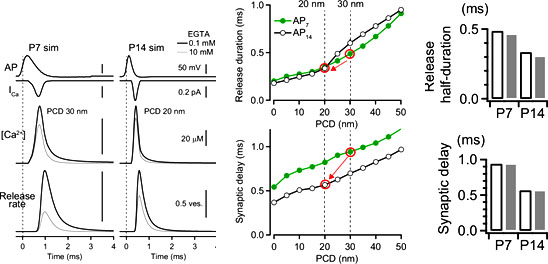
Following data are simulated using our perimeter release model. It indicates that both the release time and synaptic delay is proportional to the perimeter coupling distance. As the distance become shorter from 30 at P7 to 20 nm at P14, release time becomes shorter. Synaptic delay also becomes shorter because of two reasons, one is a shortening of coupling distance and the other is a shortening of AP duration.
The bar graphs indicate the developmental changes of synaptic delay and release duration produced from our perimeter coupling model that is similar to the experimental results, supporting the robustness of our model.
In conclusion, in the perimeter release model, tens of 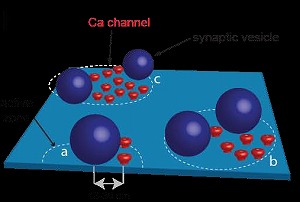
nanometer coupling distance arrangement between Ca2+ channel cluster perimeter and vesicle Ca2+ sensor allows
fast and precise neurotransmitter release, independently of the number of channels in a cluster at the calyx of Held.
Since Ca2+ channels form clusters also at cerebral and cerebellar cortical presynaptic terminals, this model might be widely applicable to various type of synapses.
(2) Calyx-type giant synapse formed in dissociated culture (Dimitrov et al, manuscript under revision).
The giant synapse calyx of Held visualized in rodent brainstem slice is an excellent preparation for studying presynaptic mechanisms, but it has some limitations including difficulty of molecular manipulations and inadequate transparency for imaging vesicles and presynaptic molecules. To break through these technical problems, we developed a calyx-like giant synapse preparation in dissociated culture. From newborn mice pups, we isolated tissues from cochlear region for presynaptic neurons and MNTB regions for postsynaptic neurons. After GFP-labeling presynaptic neurons, we co-cultured them in media containing various ingredients. After 10 days in culture GFP-labeled cochlear neurons extended their axons and formed calyx-like terminals surrounding postsynaptic neurons. These terminals underwent functional maturation and neurotransmitter release started after 15 days in vitro (DIV). They also underwent structural maturation with initial ring-like structures transformed later to finger-like structures attached with many swellings, like those described for the calyx of Held in vivo. To these calyx-like synapses in culture, we made it possible to apply imaging techniques thereby studying vesicle exocytosis and endocytosis using FM dye and transfected pHluorin, and monitoring vesicle trafficking using CypHer or quantum dot fluorescent labelling. Furthermore, we made it possible to perform simultaneous patch clamp recording with imaging from the cultured calyceal terminals. This has enabled us to monitor membrane capacitance change together with vesicular pH changes with pHluorin.
While searching for factors which promote calyx synapse formation, we found that neurotrophin 3 (NT3) and its receptor TrkC are essential, specifically for the calyx-like giant synapse formation, since their neutralizing antibodies markedly reduced calyx formation without affecting puncta-like synapse formation. NT3 antibody also inhibited structural maturation of calyx terminals. Apart from the NT3 binding domain, the NT3 receptor Trk C has a leucine-rich repeat (LRR) domain that reportedly plays essential roles in maturation of synaptic molecules. In cultured calyx synapses, we found that the LRR-specific antibody inhibited developmental synaptic elimination, i.e. from multiple to single innervation, and functional maturation in transmitter release probability, whereas NT3 antibody had no such effect. Thus, in cultured calyx synapses, structural maturation requires NT3 binding to TrkC, but synaptic elimination and functional maturation are likely mediated by LRR domain of TrkC.
(3)Vesicle imaging in cultured calyx terminal (Guillaud et al, manuscript in preparation).
After conjugating quantum (Q) dot with synaptotagmin 2 antibody, we loaded it into recycling vesicles via vesicle endocytosis. This enabled us to monitor vesicle trafficking in the calyx terminal. The Q dot labeled vesicles moved in resting terminal with irregular speeds and various trajectories. Vesicles traveling a longer distance generally had a higher maximal velocity. Microtubule disassembly using nocodazole preferentially reduced long/fast traffics, suggesting that microtubules are involved in long and fast vesicle traffickings. This type of trafficking was most prominent in immature calyx structures. When massive vesicle exocytosis was induced by bath-application of high [KCl] solution, the number of labeled vesicles markedly decreased and movements of remaining vesicles were restricted, as if vesicles were docked at the release sites. The diffusion coefficient of vesicles in cultured calyx terminals was comparable to those reported for goldfish or lizard retinal cells, but 1-3 orders of magnitude faster than that reported for frog neuromuscular junction or rat hippocampal boutons.
(4) Primary targets of diseases caused by excess endogenous presynaptic proteins
Tau is a microtubules stabilizing protein normally present in axons and axon terminals. Its overexpression is observed in tauopathies including Alzheimer’s disease. It has been recently reported that tau loading into squid giant synapse attenuates synaptic transmission (Moreno et al, 2011). By making simultaneous whole cell recording from presynaptic terminal and postsynaptic neuron at the calyx of Held synapse in slices of rodent brainstem, we tested if excess tau might affect synaptic transmission. Human recombinant tau, when loaded into calyx terminal at 10-20 μM via pipette perfusion, gradually but significantly reduced EPSC amplitude. Analysis of EPSC amplitude during and after tetanic stimulation indicated a reduction in the size of readily releasable pool (RRP) of synaptic vesicles and that the recovery of RRP after depletion was delayed in the presence of tau. To identify the target of excess tau, we further made membrane capacitance measurements and found that intra-terminal tau loading blocks vesicle endocytosis with little immediate effect on ICa or exocytic Cm. Block of endocytosis by tau explains a slow decline of synaptic transmission associated with a decrease in RRP size.
Given that the main role of tau is to stabilize microtubules, we asked whether the microtubules stabilizing drug taxol might reproduce the effect of tau on neurotransmission. Indeed, taxol, when loaded into calyceal terminals, attenuated synaptic transmission, slowed recovery from synaptic depression, and most importantly blocked vesicle endocytosis. In contrast, a tau mutant lacking the microtubules binding domain, when loaded into calyceal terminals, had no effect on neurotransmission. In vitro tubulin assembly assay confirmed that tau and taxol slowly assembled tubulin into microtubules, whereas the tau mutant had no effect. These results together suggest that excess tau over assembles microtubules, thereby blocking vesicle endocytosis as a primary target (Hori et al, manuscript in preparation).
α‐synuclein is an endogenous protein normally attached to vesicular or plasma membranes in the nerve terminal. Excessα α‐synuclein is known to accompany α‐synucleinopathy including Parkinson’s disease. We have loaded α‐synuclein into calyx terminals in acute slices and found that it slows endocytosis with no immediate effect on exocytosis or ICa. This inhibitory effect is much weaker than that observed for tau loading, but is comparable to that observed after presynaptic application of a PKG inhibitor (Eguchi et al, 2012). In fact, like PKG inhibitor, excess α‐synuclein disrupted fidelity of high frequency neurotransmission. Surprisingly, the inhibitory effect of α‐synuclein was completely rescued by nocodazole co-loaded into terminals, suggesting that excess microtubules are involved in the effect of α‐synuclein overloading. As previously reported both tau and α‐synuclein assembled microtubules in vitro (Eguchi et al, manuscript under revision).
4. Publications
4.1 Journals
Nakamura, Y., Harada, H., Kamasawa, N., Matsui, K., Rothman, J. S., Shigemoto, R., Silver, R. A., DiGregorio, D. A., Takahashi, T.
"Nanoscale Distribution of Presynaptic Ca2+ Channels and Its Impact on Vesicular Release during Development." Neuron 85(1): 145-158.
Guillaud, L., Gray, K. T., Moroz, N., Pantazis, C., Pate, E., Kostyukova, A. S.
"Role of tropomodulin's leucine rich repeat domain in the formation of neurite-like processes." Biochemistry 53(16): 2689-2700.
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
Oral Presentation
Eguchi, K. “The regulatory mechanisms of synaptic vesicle dynamics in central nervous system.” The meeting for the research on sense information processing. Hotel Centraza Hakata, Fukuoka. June 28, 2014
Eguchi, K. and Takahashi, T. “The retrograde regulation of synaptic vesicle endocytosis at CNS synapses.” The functional dynamics of synapses and neural networks, NIPS meeting. National Institute for Physiological Sciences (NIPS), Okazaki, Aichi, Japan. December 2-3, 2014.
Poster Presentation
Eguchi, K., Taoufiq, Z. and Takahashi, T. “Rho-kinase accelerates synaptic Vesicle endocytosis by modulating phosphatidylinositol-4,5-bisphosphate.” 9th FENS forum of Neuroscience. Milano Congressi, Milan, Italy. July 5-9, 2014
Guillaud, L., Dimitrov, D., Takahashi, T. “ Microtubule-dependent trafficking of synaptic vesicle “superpool” in cultured giant synapse.” The 44th annual meeting of the Society for Neuroscience. Washington convention center, Washington, D.C. November 15-19, 2014
Ohmachi, M. and Takahashi, T. “ Real-time fluorescence imaging of quantum dot-loaded single synaptic vesicles.” The 37th Annual Meeting of the Japan Neuroscience Society. Pacifico Yokohama, Japan. September 11-13, 2014
Ohmachi, M. and Takahashi, T. “ Real-time fluorescence imaging of quantum dot-loaded single synaptic vesicles.” The 52nd Annual Meeting of the Biophysical Society of Japan. Sapporo Convention Center, Sapporo, Hokkaido, Japan. September 25-27, 2014
Hori, T., Rigby, M., Takahashi, T. “The recycling pool size estimated by different stimulation frequencies at the calyx of Held presynaptic terminal.” The 92nd Annual Meeting of the Physiological Society of Japan. Kobe Convention center, Kobe, Japan. March 21-23, 2015
5. Intellectual Property Rights and Other Specific Achievements
5.1 Grant:
Kohgaku Eguchi: Grand-in-Aid for Young Scientists B, KAKENHI (2012-2015).
6. Meetings and Events
6.1 16th International Neuroscience Winter Conference
Date: April 10, 2014
Venue: Hotel Das Central, Sölden, Austria
Speaker: Tomoyuki Takahashi
Title: Perimeter Ca2+ channel coupling to transmitter release at developing calyces of Held
6.2 Nikon Imaging Center at Harvard Medical School Imaging Symposium
Date: May 2, 2014
Venue: Harvard Medical School, Boston, U.S.A.
Speaker: Tomoyuki Takahashi
Title: Imaging presynaptic functions at the calyx of Held
6.3 CNRS - Jacques-Monod Conference “Optical imaging of brain structure and function on multiple spatial scales”
Date: June 11-15, 2014
Venue: C.N.R.S. - Station Biologique de Roscoff, France
Speaker: Tomoyuki Takahashi
Title: Imaging vesicle trafficking in calyceal presynaptic terminals in culture
6.4 NIH Neuroscience Seminar Series.
Date: January 12, 2015
Venue: National Institutes of Health (NIH), Maryland, U.S.A.
Speaker: Tomoyuki Takahashi
Title: Visiting factories in the nerve terminal.
7. Other events
7.1 Technical seminar by the 16th Brain Science Meeting
Date: June 28-29, 2014
Venue: Okinawa Kariyushi Urban Resort Naha, Okinawa
Speaker: Hiroshi Takagi
Title: Trial to analyze a synapse function at a micro and a macro level
7.2 Teaching class ‘Super resolution microscopy in life sciences: imaging beyond the diffraction limit’ Part of the 'Emerging technologies in life sciences'
For the first year OIST Ph.D. students, organized by Professor Ichiro Maruyama, OIST
Date: July 25, 2014
Speaker: Laurent Guillaud
7.3 Onna-village/OIST Children’s School of Science 2014
Date: August 17-18, 2014
Venue: Fureaitaiken Center, Onna-village
Speaker: Hiroshi Takagi
Topic: "Brain and Robot" for Elementary school 5th -6th Grade
7.4 Career talk, OIST mini café in Tokyo
Date: April 25, 2014
Venue: Goblin.Park, Tokyo
Speaker: Setsuko Nakanishi
7.5 Science café at OIST Open campus
Date: February 1, 2015
Venue: Okinawa Institute Science and technology Graduate University
Speaker: Zacharie Taoufiq
Title: "Will we be able to cure all brain cognitive and memory diseases during the 21st century?"



