FY2012 Annual Report
Cellular and Molecular Synaptic Function Unit
Professor Tomoyuki Takahashi
Abstract
In the neuronal system, dynamic changes of synaptic strength play critical roles in switching functional neuronal circuits. Regarding synaptic strength, compared with postsynaptic mechanisms much less is known for presynaptic mechanisms, primarily because of small nerve terminal structures preventing applications of electrophysiological and imaging techniques. The calyx of Held is a giant glutamatergic nerve terminal visually identified in the mammalian auditory brainstem slices. This fast relay synapse undergoes dramatic developmental changes in its structure, functional properties and molecular compositions during the second postnatal (P) week, when rodents start to hear sound at P10-12. By applying molecular, imaging and patch-clamp techniques to this synapse of developing rodents, we aim at elucidating presynaptic regulatory mechanisms underlying synaptic transmission. Our progress in the fiscal year 2012 is as follows:
(1) First determination of refilling speed of vesicles with neurotransmitter (Hori T & Takahashi T, 2012 Neuron)
Chemical neurotransmitter is stored in synaptic vesicles. Upon an electrical signal reaching the synapse, the vesicles fuse into the nerve terminal membrane, causing the vesicle membrane to break open and neurotransmitter to be released. The empty vesicles then return to their original nerve terminal and are refilled with neurotransmitter to be reused for another round of synaptic transmission – a recycling mechanism that enables long-lasting synaptic transmission. To maintain synaptic transmission, the vesicles must be continually refilled with neurotransmitter. The rate at which vesicles refill is an important parameter that limits the speed at which continuous neurotransmission can occur – or how accurately actions or thoughts can be processed and how long they can be maintained. However, until now there has been no reliable method for measuring vesicle-refilling rates. Hori and Takahashi have designed a technique for monitoring vesicle refilling in a living neuron within a mouse brain using the neurotransmitter glutamate and found that it takes roughly 15 seconds for empty vesicles to be refilled with the neurotransmitter. They also discovered that this refilling speed depends upon chloride concentrations within the nerve terminal with the speed decreasing when the chloride concentration deviates away from a normal level of 10-30 mM. Not only has this study made a fundamental contribution to understanding synaptic transmission, but it could also provide clues for finding molecular targets in neuronal disorders.
(2) Rho-kinase accelerates synaptic vesicle endocytosis by linking PKG activity to PIP2 synthesis (Taoufiq Z, Eguchi K & Takahashi T, Journal Neuroscience in press)
1. Staff
- Dr. Tomoyuki Takahashi, Professor
- Dr. Hiroshi Takagi, Group Leader
- Dr. Kohgaku Eguchi, Researcher
- Dr. Tetsuya Hori, Researcher
- Dr. Yukihiro Nakamura, Researcher (until September, 2012)
- Dr. Laurent Guillaud, Researcher
- Dr. Setsuko Nakanishi, Researcher
- Dr. Zacharie Taoufiq, Researcher
- Dr. Masashi Ohmachi, Researcher (from January, 2013)
- Dr. Dimitar Dimitrov, Technician
- Ms. Kaori Egashira, Research Administrator (until December, 2012)
- Ms. Rieko Uezu, Research Administrator (until January, 2013)
- Ms. Sayori Gordon, Research Administrator (from February, 2013)
2. Collaborations
Research theme: Regulatory mechanisms for transmitter release
- Type of collaboration: Joint research
- Name of partner organization: Doshisha University, Faculty of Life and Medical Sciences
- Principal Researcher: Tomoyuki Takahashi, OIST., Doshisha University, Graduate School of Brain Science & Faculty of Life and Medical Sciences
- Researcher:
■ Naoto Saitoh, Doshisha University
■ Takafumi Miki, Doshisha University
Research theme: Developmental changes in the presynaptic calcium transient profiles associated with transmitter release
- Type of collaboration: Scientific Collaboration
- Name of partner organization: Pasteur Institute
- Principal Researcher: David DiGregorio, Pasteur Institute
- Researcher: David DiGregorio, Pasteur Institute
Research theme: Developmental changes in the presynaptic calcium channels
- Type of collaboration: Scientific Collaboration
- Name of partner organization: National Institute for Physiological Sciences, Department of Cerebral Research, Division of Cellular Strucure
- Principal Researcher: Ryuichi Shigemoto, NIPS(National Institute for Physiological Sciences)
- Researcher:
■ Ryuichi Shigemoto, NIPS(National Institute for Physiological Sciences)
■ Ko Matsui, NIPS(National Institute for Physiological Sciences)
3. Activities and Findings
3.1 Kinetics of Synaptic Vesicle Refilling with Neurotransmitter Glutamate (Hori & Takahashi, 2012 Neuron)
After exocytic release of transmitter, synaptic vesicles are retrieved by endocytosis and recycled to be reused. At glutamatergic excitatory synappses imaging studies using fluorescence indicators have revealed different types of vesicle endocytosis with different kinetics, ranging from subsecond to tens of seconds. It has been argued that fast endocytosis and recycling are required for small synapses with limited number of releasable vesicles. Despite the wealth of knowledge on the endocytic kinetics, little is known for the time required for vesicles to be refilled with neurotransmitter. This information is important because empty vesicles do not contribute to neurotransmission. In isolated or reconstructed vesicles, the uptake time constant of the neurotransmitter glutamate goes beyond several minutes. This time course is far too slow to account for the recovery of synaptic transmission after depletion of releasable vesicles. Clearly, the original kinetics of glutamate uptake into vesicles is distorted during the procedure of vesicle isolation or reconstruction.
It is therefore desirable if one can measure the time of vesicular glutamate uptake in situ. We challenged this problem using the calyx of Held presynaptic terminal. In this terminal, whole cell wash out of cytosolic glutamate leads to a depletion of glutamate in vesicles, because of vesicle recycling combined with a passive leakage. Glutamate depletion from vesicles results in a reduction of the frequency and mean amplitude of spontaneous miniature, i.e., vesicular, EPSCs and mean amplitude of nerve-evoked EPSCs (Ishikawa et al, 2002 Neuron). In contrast, injection of glutamate into the terminal increases vesicular glutamate content and the size of EPSCs. To address the question on the kinetics of vesicle refilling with glutamate, we introduced the caged glutamate compound MNI-glutamate (2-10 mM) into the terminal.
When glutamate was washed out from the terminal, the EPSC amplitude declined to a low level (<20%, Fig 1A). Application of an UV flash light (1s) caused a recovery of EPSCs to the original amplitude (>95%). This recovery was completely abolished in the presence of bafilomycin in the bath solution (Fig 1B). Bafilomycin blocks acidification of vesicles by vacuolar type H+-ATPase that is required for glutamate uptake with vesicular glutamate transporters (VGLUTs). This recovery was not associated with changes in the membrane capacitance (Fig 1C), suggesting that glutamate uncaging had no effect on exocytosis of vesicles. Furthermore, glutamate uncaging increased the frequency and amplitude of miniature EPSCs (Fig 1D), suggesting that vesicles were refilled with glutamate photo-released from the MNI compound.
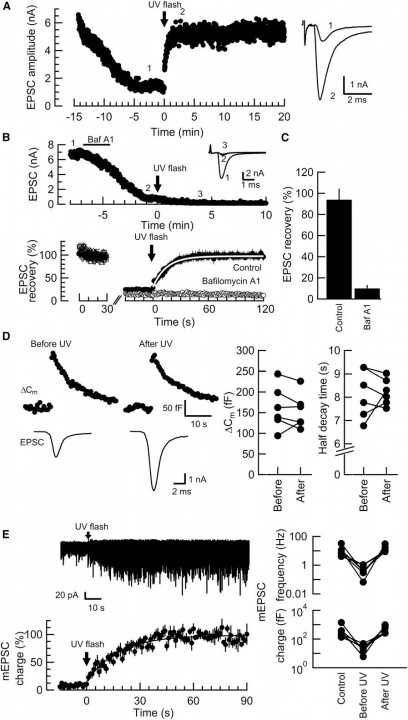
Fig 1
From these data we analyzed the kinetics of glutamate uptake. To gain information as to the glutamate concentration raised by glutamate uncaging, we loaded given concentration of glutamate into calyces and measured the EPSC amplitude (Fig 2A). We then uncaged glutamate from the MNI compound at different concentrations (2-10 mM, Fig 2B). By normalizing the maximal EPSC amplitude between glutamate loading and MNI-glutamate uncaging, we obtained the relationship between the time constant of EPSC recovery and glutamate concentrations (Fig 2C). Lineweaver-Burk plot analysis of these data indicated that the maximal glutamate uptake rate (Vmax) was 15 sec and the glutamate affinity of the transporter (Km) was 0.9 mM. The latter value is similar to what have been reported for VGLUT1 and VGLUT2.
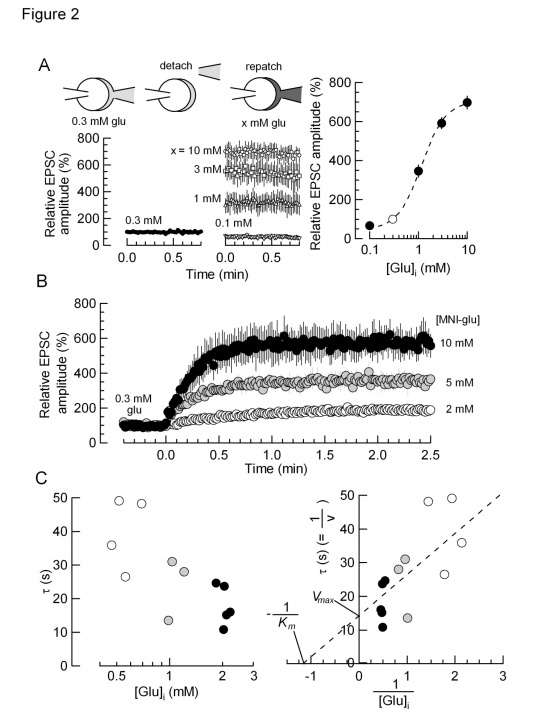
Fig 2
The 15s time constant measured for glutamate refilling into vesicles is fast enough to fill up vesicles in the CME pathway (Fig 3), but it is too slow to fill vesicles undergoing fast recycling pathways such as the “kiss-and-run” fusion flicker that is thought to have subsecond time constant. In order to contribute to synaptic transmission, vesicles after recycling through fast pathways must wait for them refilled with transmitter before reused. In fact, it has been estimated that “kiss-and run” vesicles are reused only after 23 sec. This time course is not much different from that of CME. Thus, it remains to be re-examined whether the fast recycling mechanisms have physiological significance.
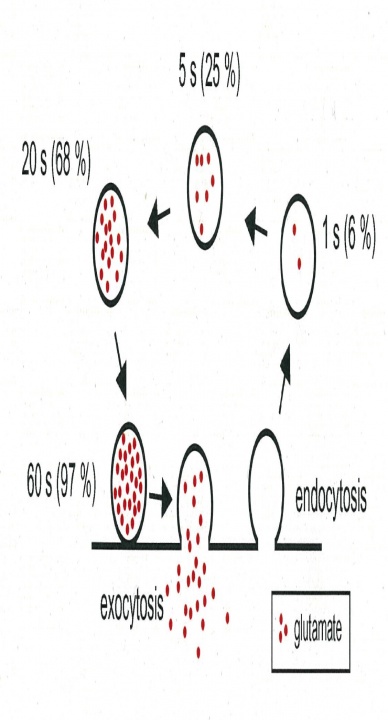
Fig 3
In isolated vesicles, glutamate uptake is known to have a biphasic dependence of Cl- concentrations. Presynaptic terminals including the calyx of Held possess Cl- -permeable channels such as glycine receptors, therefore intra-terminal Cl- concentration can change from time to time. We tested whether glutamate refilling depends upon Cl- at the calyx of Held. When [Cl-] was reduced below mM, the rate and magnitude of glutamate refilling was markedly reduced. When [Cl-] was increased above 100 mM, the rate and magnitude of glutamate refilling was significantly slowed. Furthermore, when [Cl-] was decreased below 10 mM the rate and magnitude of glutamate refilling was significantly slowed (Fig 4). Thus, these results in situ agree with those previously reported with isolated vesicles.
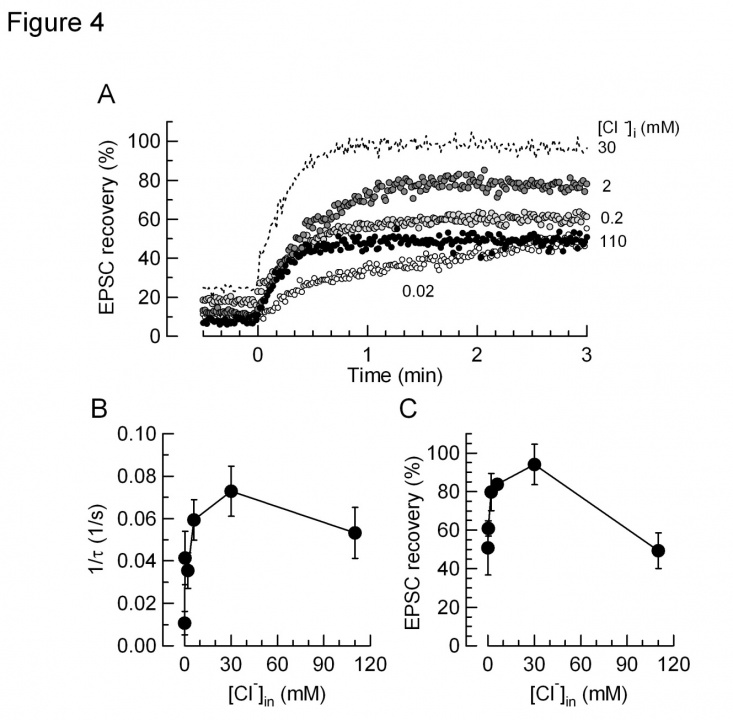
Fig 4
If vesicle refilling time limits the time for vesicle reuse, manipulations to retard the refilling rate will cause defects in neurotransmission. We are currently studying this issue.
3.2 Rho-kinase accelerates synaptic vesicle endocytosis by linking PKG activity to PIP2 synthesis (Taoufiq Z, Eguchi K & Takahashi T, under review)
Rho-kinase plays diverse roles in cell motility. During neuronal development, Rho-kinase is involved in neuronal migration, and in neurite outgrowth and retraction. Rho-kinase remains highly expressed in mature neurons, but its physiological roles are poorly understood. Taoufiq et al report that Rho-kinase plays a key role in the synaptic vesicle recycling system in presynaptic terminals. Vesicles consumed by excessive exocytosis are replenished by accelerating vesicle endocytosis via a retrograde feedback mechanism involving nitric oxide released from postsynaptic cells. This homeostatic control system involves presynaptic cyclic GMP-dependent protein kinase (PKG) and a plasma membrane phospholipid, phosphatidylinositol-4,5-bisphophate (PIP2) (Eguchi et al, 2012 Neuron). Taoufiq et al found that application of a Rho-kinase inhibitor, a PKG inhibitor or both, reduced the PIP2 content in Wistar rat brainstem synaptosomes to a similar extent. Likewise, application of the Rho-kinase inhibitor into the calyx of Held presynaptic terminal slowed vesicle endocytosis to the same degree as did application of the PKG inhibitor. This endocytic slowing effect of the Rho-kinase inhibitor was cancelled by co-application of PIP2 into the terminal. By contrast, a RhoA activator increased the PIP2 content and reversed the effect of the PKG inhibitor in brainstem synaptosomes. The RhoA activator, when loaded into calyceal terminals, also rescued the endocytic slowing effect of the PKG inhibitor. Furthermore, intra-terminal loading of anti-PIP2 antibody slowed vesicle endocytosis and blocked the rescuing effect of the RhoA activator. It is concluded that Rho-kinase links presynaptic PKG activity to PIP2 synthesis, thereby controlling the homeostatic balance of vesicle exocytosis and endocytosis in nerve terminals.
4. Publications
4.1 Journals
- Hori, T. & Takahashi, T. Kinetics of synaptic vesicle refilling with neurotransmitter glutamate. Neuron 76, 511-517, doi:10.1016/j.neuron.2012.08.013 (2012).
- Eguchi, K., Nakanishi, S., Takagi, H., Taoufiq, Z. & Takahashi, T. Maturation of a PKG-dependent retrograde mechanism for exoendocytic coupling of synaptic vesicles. Neuron 74, 517-529, doi:10.1016/j.neuron.2012.03.028 (2012).
- Arama, J., Boulay, A. C., Bosc, C., Delphin, C., Loew, D., Rostaing, P., Amigou, E., Ezan, P., Wingertsmann, L., Guillaud, L., Andrieux, A., Giaume, C. & Cohen-Salmon, M. Bmcc1s, a novel brain-isoform of Bmcc1, affects cell morphology by regulating MAP6/STOP functions. PLoS One 7, e35488, doi:10.1371/journal.pone.0035488 (2012).
- Moroz, N., Guillaud, L., Desai, B. a. K., A. & contribution), e. Mutations changing tropomodulin affinity for tropomyosin alters neurite formation and extension. PeerJ, doi:10.7717/peerj.7 (2013).
- Takagi, H., Setou, M., Ito, S. & Yao, I. SCRAPPER regulates the thresholds of long-term potentiation/depression, the bidirectional synaptic plasticity in hippocampal CA3-CA1 synapses. Neural Plast 2012, 7 pages, doi:10.1155/2012/352829 (2012).
- Zhou, R., Niwa, S., Guillaud, L., Tong, Y. & Hirokawa, N. A Molecular Motor, KIF13A, Controls Anxiety by Transporting the Serotonin Type 1A Receptor. Cell Rep 3, 509-519, doi:10.1016/j.celrep.2013.01.014 (2013).
4.2 Books and other one-time publications
Takahashi, T., Hori, T., Nakamura, Y., Yamashita, T.
Patch-Clamp Recording Method in Slices for Studying Presynaptic Mechanisms.
Patch Clamp Techniques: From Beginning to Advanced Protocols (ed. Okada, Y.) 137-145
(Springer Verlag, Japan, 2012).
4.3 Oral and Poster Presentations
-
Taoufiq, Z., Eguchi, K. & Takahashi, T. Rho-kinase accelerates vesicle endocytosis in a presynaptic terminal by linking PKG Activity to phosphatidylinositol-4,5-bisphophate synthesis., International Symposium, Mechanisms of synaptic transmission., Doshisha University, Kyoto, Japan., December 6-7, 2012.
-
Pantazis, C., Guillaud, L., Moroz, N. & Kostyukova, A. Structural isoform-specific differences in tropomodulin and their implication in neuronal differentiation. Keystone Symposia – Structural Biology of Cellular Processes: From Atoms to Cells., Keystone, Colorado, USA., January 22-27, 2012.
-
Nakamura, Y., Harada, H., Kamasawa, N., Matsui, K., Shigemoto, R., Rothman, J. S., Silver, R., David, A., Gregorio, D. & Takahashi, T. Developmental changes in spatial profiles of Ca2+ channel and Ca2+ influx underlying tightening of Ca2+-secretion coupling at the calyx of Held. CNRS Jacques Monod Conference, “Imaging neuronal functions: from molecules to circuits”, Roscoff, France., July 2, 2012.
-
Hori, T. & Takahashi, T. Neurotransmitter refilling rate is a rate-limiting step for synaptic transmission at the excitatory synapse. Neuroscience 2012, the 35th Annual Meeting of the Japan Neuroscience Society., Nagoya, Japan. September 18-21, 2012.
-
Guillaud, L. & Takahashi, T. Mobility of synaptic vesicles in cultured giant presynaptic terminals. International Joint Meeting of the German Society for Cell Biology and German Society for Developmental Biology., Heidelberg, Germany., March 20-23, 2012.
-
Guillaud, L., Dimitrov, D. & Takahashi, T. Novel cellular model and methods to study synaptogenesis and synaptic transmission., The 2nd International workshop – Toward the development of a R&D cluster in Okinawa. , Okinawa Institute of Science and Technology. Okinawa, Japan., March 29-30, 2012.
-
Guillaud, L., Dimitrov, D. & Takahashi, T. Morphological development and maturation of calyx of Held-like synapses in primary culture., Neuroscience 2012, the 35th Annual Meeting of the Japan Neuroscience Society., Nagoya, Japan., September 18-21, 2012.
-
Guillaud, L., Dimitrov, D. & Takahashi, T. Synaptic vesicle dynamics and trafficking in the developing calyx of Held culture., Neuroscience 2012, the 42nd Annual Meeting of the Society for Neuroscience, New Orleans, LA, USA., October 13-17, 2012.
-
Guillaud, L., Dimitrov, D. & Takahashi, T. Developmental change in synaptic vesicle trafficking at the calyx of Held., International Symposium, Mechanisms of synaptic transmission., Doshisha University, Kyoto, Japan., December 6-7, 2012.
-
Eguchi, K., Taoufiq, Z. & Takahashi, T. PKG accelerates vesicle endocytosis via Rho-associated protein kinase at the calyx of Held synapse., The 90th Annual Meeting of the Physiological Society of Japan., Tokyo, Japan., March 27-29, 2012.
-
Dimitrov, D., Guillaud, L., Takagi, H., Saitoh, N. & Takahashi, T. Dissociated primary culture of the giant synapse calyx of Held as a novel in vitro cell model., International Symposium, Mechanisms of synaptic transmission., Doshisha University, Kyoto, Japan., December 6-7, 2012.
-
Dimitrov, D., Guillaud, L., Saitoh, N., Takagi, H. & Takahashi, T. Culture preparation of the calyx of Held giant synapse - a novel in vitro cell model., Neuroscience 2012, the 35th Annual Meeting of the Japan Neuroscience Society., Nagoya, Japan., September 18-21, 2012.
-
Dimitrov, D., Guillaud, L., Saitoh, N., Takagi, H., Eguchi, K. & Takahashi, T. Primary culture of the giant synapse calyx of Held - A novel in vitro cell model., Neuroscience 2012, the 42nd Annual Meeting of the Society for Neuroscience., New Orleans, LA, U.S.A., October 13-17, 2012.
-
Eguchi, K. Synaptic transmission between neurons and vesicle dynamics: thestudy of PKG-dependent retrograde mechanisms for vesicle endocytosis and its developmental change., Kyutech Neuroscience seminar, Kyushu Institute of Technology, Fukuoka, Japan., June, 2012.
-
Eguchi, K., Nakanishi, S., Taoufiq, Z., Takagi, H. & Takahashi, T. Postnatal maturation of a PKG-dependent retrograde mechanism for vesicle endocytosis at the calyx of Held., International Symposium, Mechanisms of synaptic transmission., Doshisha University, Kyoto, Japan., December 6-7, 2012.
5. Intellectual Property Rights and Other Specific Achievements
5.1 Grant:
Kohgaku Eguchi: Grant-in-Aid for Young Scientists B, KAKENHI (2012-2015).
6. Meetings and Events
6.1 Conference Invited Lecture
Title: Developmental change of the presynaptic vesicle regulatory mechanism in the central
nervous system
● Conference name: JST PRESTO PROJECT,Development and Function of Neuronal Networks
The 6th PRESTO Meeting of the Research Area
● Date: June 23, 2012.
● Venue: Okinawa, Japan
● Speaker: Takahashi, T.
Title: Postnatal development of the mechanism for the maintenance of central synaptic transmission.
● Conference name: Neuroscience 2012, the 35th Annual Meeting of the Japan Neuroscience
Society.
● Date: September 18-21, 2012.
● Venue: Nagoya, Japan.
● Speaker: Takahashi, T.
Title: Vesicle reuse for the maintenance of synaptic transmission.
● Conference name: Cellular Molecular Mechanisms of Sensory Processing
● Date: October 9, 2012
● Venue: Gottingen, Germany.
● Speaker: Takahashi, T.
Title: Recycling Maintenance of Hi-Fi Synaptic Transmission in International Symposium, Mechanisms of synaptic transmission.
● Conference name: International Symposium, Mechanisms of synaptic transmission.
● Date: December 6-7, 2012.
● Venue: Doshisha University, Kyoto, Japan.
● Speaker: Takahashi, T.
6.2 Seminar
Title: Synaptic vesicles – The butlers of the brain.
● Date: June 15, 2012 .
● Venue: Okinawa Institute of Science and Technology Graduate University, Okinawa, Japan
● Speaker: Guillaud, L.
Title: MPI Seminar: Synaptic vesicle mobility in cultured calyx of Held.
● Date: March 18, 2013
● Venue: Max Plank Institute for Biophysical Chemistry,
Department of Membrane Biophysics, Germany.
● Speaker: Guillaud, L.
Title: FMP Colloquium: Mobility of synaptic vesicles in cultured calyx of Held.
● Date: March 26, 2013
● Venue: Leibniz Institute for Molecular Pharmacology, Berlin, Germany.
● Speaker: Guillaud, L.
Title: CCO Semiar: Spatio-temporal analysis of single synaptic vesicle in cultured calyx of Held.
● Date: March 27, 2013
● Venue: Charite Cross Over-Neurocure, Berlin, Germany.
● Speaker: Guillaud, L.



