Takebayashi Group
Open positions
-
I have an opening of fully funded postdoc position, starting from January, 2025 or later. Please contact me if you are interested in studying organometallic chemistry and catalysis. (Posted September 30th, 2024)
-
I am looking for postdocs with independent funding, such as JSPS and Canon fellowships. Those who are interested in organometallic chemistry and homogeneous catalysis, please contact me for more details.
Profile

Research projects
New strategies to activate dinitrogen N2
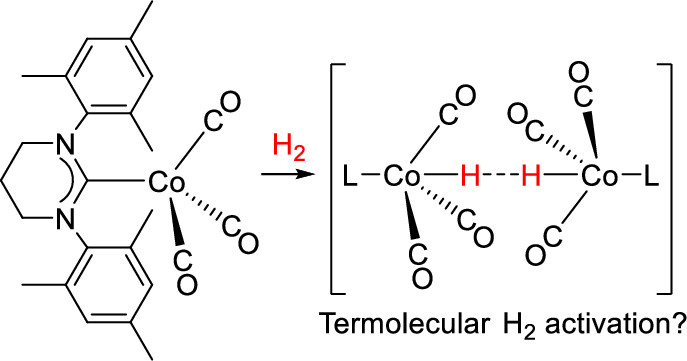
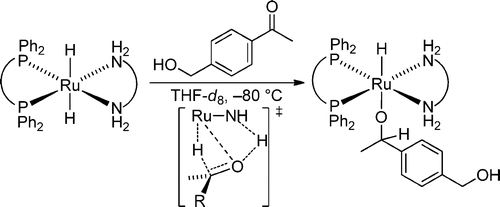
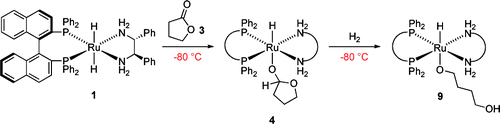
The most abundant gas on earth, dinitrogen N2, is the ultimate source of nitrogen for living organisms. Despite its abundance, N2 cannot be directly metabolized by most of the organisms due to its stability. Currently, N2 is converted industrially into biologically available form, ammonia NH3, using an energy-intensive method developed in 1909. Thus, chemists have been searching for a sustainable alternative method for decades. One of the possible alternative is the utilization of homogeneous catalysts that can be much more efficient than current heterogeneous catalyst. However, no homogeneous catalyst for the production of ammonia from N2 and molecular hydrogen H2 is known to date partly due to the lack of a coordination mode of N2 to transition metal that allows reaction with H2 and catalytic turnover. In this project, we are going to develop a new coordination mode of N2 to develop the first homogeneous hydrogenation of N2.
Sustainable chemistry with base metal catalysis
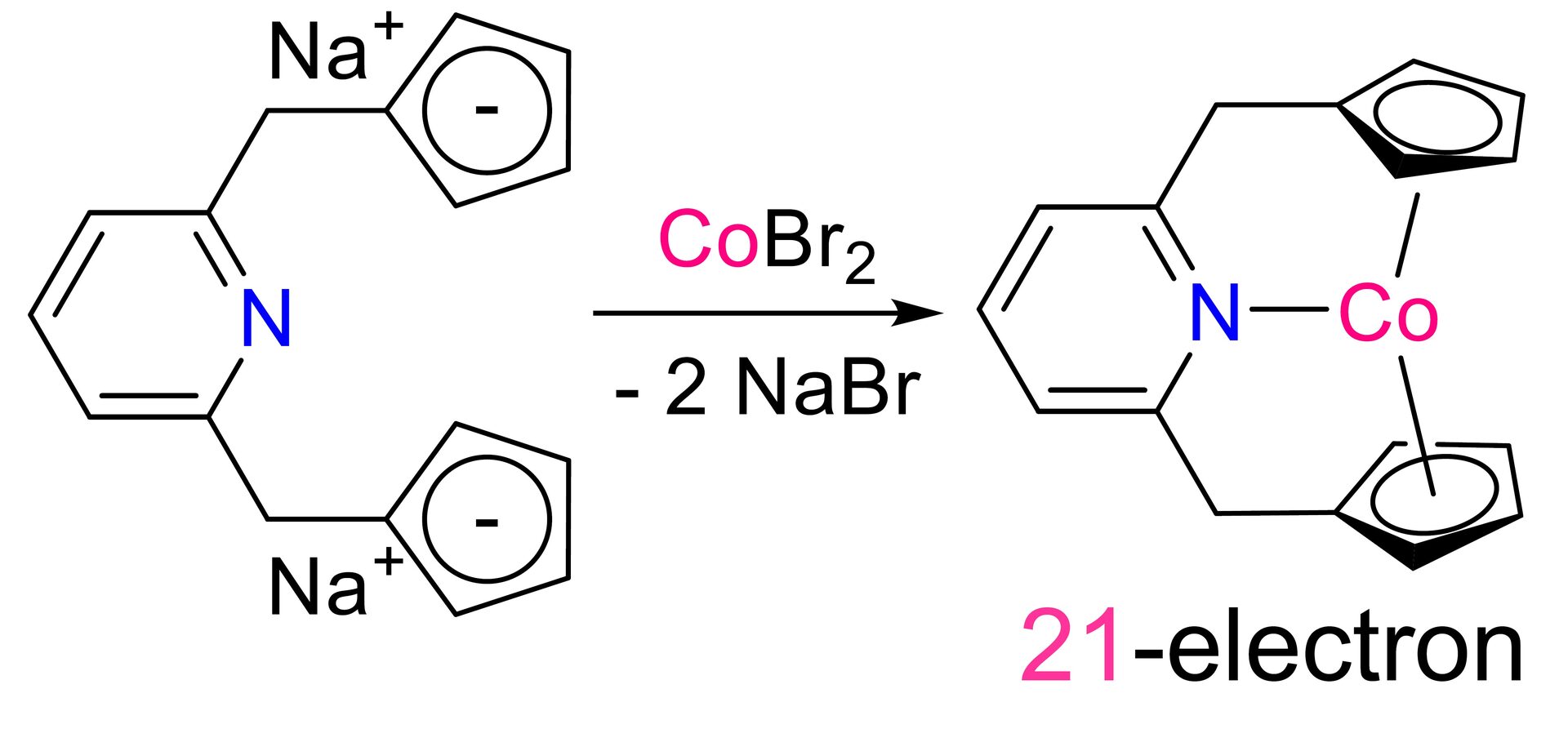
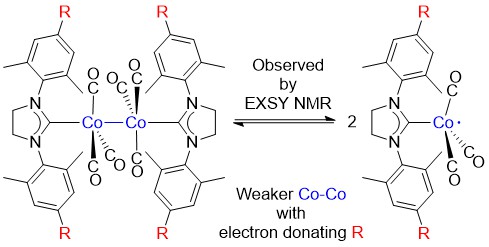
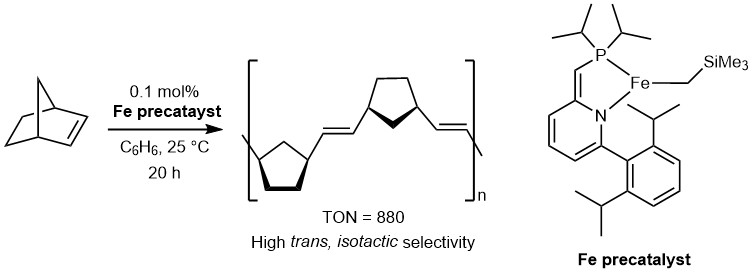
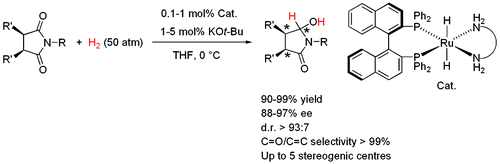
Precious metals such as, ruthenium (Ru), rhodium (Rh), palladium (Pd), iridium (Ir), and platinum (Pt), are known to form very active, and selective catalysts, and are used widely in chemical industry. However, such precious metals are costly. Furthermore, depletion of precious metals are expected in future due to ever increasing use of it. Base metals such as, molybdenum (Mo), manganese (Mn), iron (Fe), cobalt (Co), and nickel (Ni) are cheap, and abundant, however it is known to be more difficult to study as it is often paramagnetic. In this project, we are developping unprecidented catalytic reactions catalyzed by base metal complexes.
Selected publications (My Google Scholar page is here.)
Lab equipment
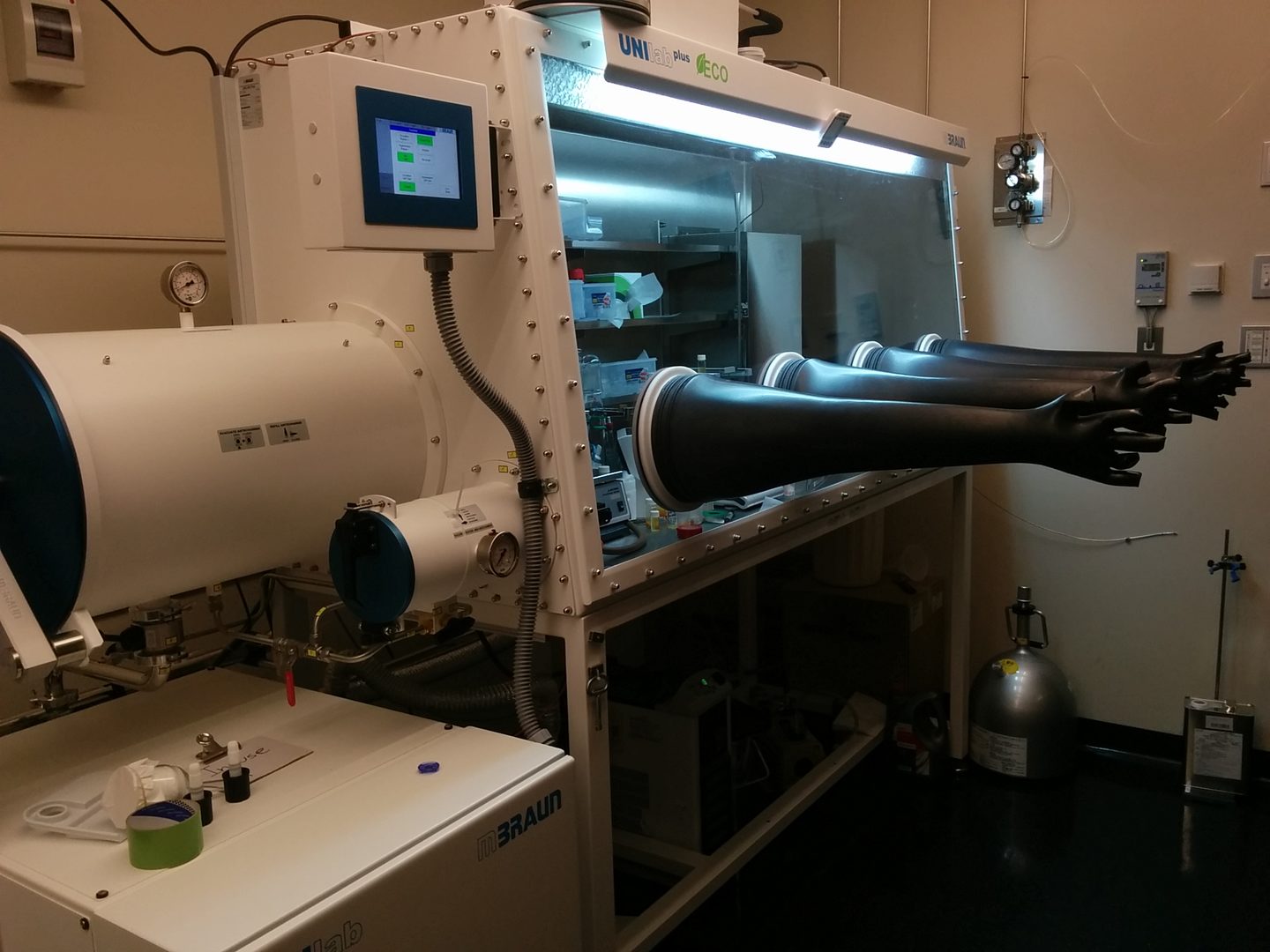
mBraun Unilabplus Glovebox with four gloves
Shimazu Gas Chromatograph GC2014 equipped with TCD (Shincarbon ST column, 2m x 3mm) and FID detectors (Astec Chiraldex® B-DM column, 30m x 0.25mm).

A 180cm width fumehood equipped with a standard Schlenk maniford, stirrers, oil baths etc.

Buchi Rotavapor R-215 system equipped with an automatic vacuum controller, and a chiller.

Thermo Scientific Nicolet iS5 FTIR spectrometer

Agilent 8860 GC/5977B MSD GCMS system