Optical Neuroimaging Unit (Bernd Kuhn)

Unit outline

Dr. Bernd Kuhn
bkuhn@oist.jp
Research Abstract
One of the key questions in neuroscience is how behavior arises from cellular activity and how information is processed in the brain. To answer these questions it is necessary to know the brain activity on different levels of organization – the activity in a single neuron, in the local neuronal network, and in the whole brain. Unfortunately, our ability to measure the neuronal activity on all of these levels is still very limited because of the complex, fine, and intermingled structure of the brain and its fast and simultaneous activity. Lately, however, genetic and optical tools were developed which allow us to have a much closer look than ever before.
Neuronal activity is indicated by membrane voltage changes in neurons followed in many cases by calcium concentration changes. Over the last decades synthetic and also genetically-encoded fluorescent probes were designed which transform changes of membrane voltage or calcium concentration into fluorescence changes, and so convert neuronal activity into an optical signal. These indicators enable us to make neuronal activity visible. Modern microscopy techniques allow us to record this optical signal.
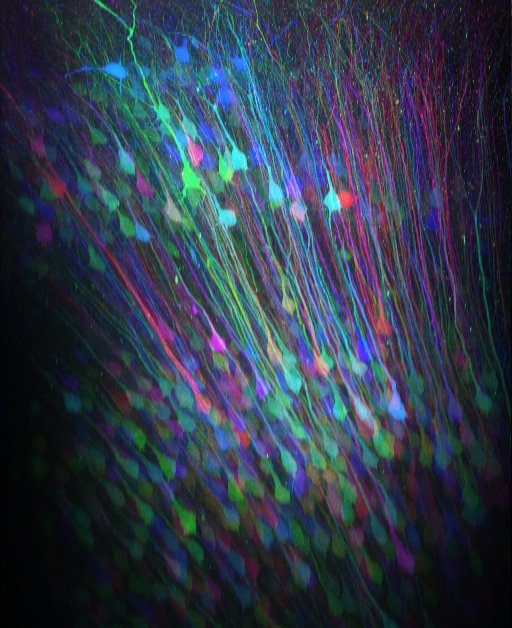
In our lab we use genetically encoded calcium indicators which can be specifically targeted to subpopulations of neurons. By combination this labeling technique with two-photon microscopy we can reconstruct cells three-dimensionally (see movie of cerebeller Purkinje cells or movie of posterior parietal cortex) and record calcium activity from cellular compartments - the soma, dendrites, and axons of neurons – almost simultaneously in the behaving animal.
For higher temporal resolution we use the synthetic voltage-sensitive dyes ANNINE-6 or ANNINE-6plus which are both pure electrochromic dyes with, therefore, a temporal resolution in the nanosecond range (10-9 s). The signals can be imaged with two-photon microscopy in vitro and in vivo. We study the mouse barrel cortex, a specialized part of the somatosensory cortex representing the large vibrissae on the snout of the mouse, and Purkinje cells of the cerebellum.
Our aim is to develop new techniques to go beyond the current limitations of signal detection in the intact brain. The tools we are developing will be applicable to many different fields in neuroscience. For example, we developed a chronic cranial window that easily allows drug testing in vivo. We hope that they will bring us a step closer to answer the questions of behavior arousal and information processing in the brain.