FY2022 Annual Report
pi-Congugated Polymers Unit
Professor Christine Luscombe
Abstract
2022 corresponds to the first full year for the pi-Conjugated Polymers Unit at OIST. We welcomed many new researchers (2 post-docs; 1 technician; hosted 6 rotation students, 2 of whom became official graduate students; hosted 3 interns and 1 Visiting Research Student). We have nearly completed setting up our lab, although we will be moving into new lab space in April 2023, which will disrupt research again.
Nevertheless, we have had a productive year. Our work has resulted in 16 peer-reviewed publications, 3 non-peer-reviewed publications, 19 conference presentations, 4 seminars, and 3 poster presentations. The work can be broadly categorized into the following categories: (i) Synthesis of materials and their fundamental studies; (ii) Mixed ionic/electronic conducting polymers; (iii) Polymers for stretchable applications; (iv) Microplastics in the environment; (v) IUPAC; (vi) Miscellaneous collaborative work. The unit received its first Kakenhi Award and received 2 SHINKA Awards.
1. Staff
As of March 31st2023
1.1. Current members
- Prof. Christine Luscombe, Professor
- Dr. Baitan Chakraborty, Postdoctoral Scholar
- Dr. Wissem Khelifi, Postdoctoral Scholar
- Dr. Samantha Phan, Postdoctoral Scholar
- Dr. Isha Sanskriti, Postdoctoral Scholar
- Dr. Preeti Yadav, Postdoctoral Scholar
- Ms. Nadege Bonnet, Technician
- Ms. Nivedha Velmurugan, Graduate Student
- Mr. Tom Tassilo Wilfling, Graduate Student
- Mr. Abdulrahman Bakry, Rotation Student
- Ms. Midori Tanahara, Research Administrator
1.2. Alumni
- Mr. Daniel Gutierrez Del Rio, Rotation Student
- Mr. Callum Hudson, Rotation Student
- Mr. Aditya Singh, Rotation Student
- Ms. Makiko Emori, Intern
- Ms. Gilda Quezada Correa, Intern
- Mr. Lorenzo Guio, Visiting Research Student
- Ms. Angela Lin, JSPS Intern
2. Collaborations
- Dr. Nicholas D’Antona, National Institute of Standards and Technology, Maryland, USA
- Dr. Lauren Asselta, National Institute of Standards and Technology, Maryland, USA
- Prof. Jiun-Tai Chen, National Yang Ming Chiao Tung University, Hsinchu, Taiwan
- Dr. Jim De Yoreo, Pacific Northwest National Laboratory, Washington, USA
- Dr. Wesley Farrell, United States Naval Academy, Annapolis, USA
- Dr. Christopher M. Fellows, University of New England, Armidale, Australia
- Dr. Lucas Flagg, National Institute of Standards and Technology, Maryland, USA
- Prof. Daniel Gamelin, University of Washington, Seattle, USA
- Prof. David Ginger, University of Washington, Seattle, USA
- Dr. Roger Hiorns, CNRS, Pau, France
- Dr. Daniel Keddie, University of Wolverhampton, Wolverhampton, UK
- Dr. Ruipeng Li, Brookhaven National Laboratory, New York, USA
- Prof. J. Devin Mackenzie, University of Washington, Seattle, USA
- Prof. Arka Majumdar, University of Washington, Seattle, USA
- Prof. John Matson, Virginia Tech, Blacksburg, USA
- Prof. Krzysztof Matyjaszewski, Carnegie Mellon University, Pittsburgh, USA
- Prof. Jan Merna, , University of Chemistry and Technology, Prague, Czech Republic
- Dr. Graeme Moad, CSIRO, Clayton South, Australia
- Prof. Tamaki Nakano, Hokkaido University, Sapporo, Japan
- Prof. Jacqueline L. Padilla-Gamiño, University of Washington, Seattle, USA
- Stanislaw Penczek, Polish Academy of Sciences, Lodz, Poland
- Dr. Lee Richter, National Institute of Standards and Technology, Maryland, USA
- Prof. Greg Russell, University of Canterbury, Christchurch, New Zealand
- Prof. Gerald Seidler, University of Washington, Seattle, USA
- Prof. Natalie Stingelin, Georgia Institute of Technology, Atlanta, USA
- Prof. Patrick Théato, Karlsruhe Institute of Technology, Karlsruhe, Germany
- Prof. Paul Topham, Aston University, Birmingham, UK
- Prof. Lydia Sosa Vargas, Sorbonne Université, Paris, France
- Prof. Dianne J. Xiao, University of Washington, Seattle, USA
3. Activities and Findings
3.1. Synthesis of materials and their fundamental studies
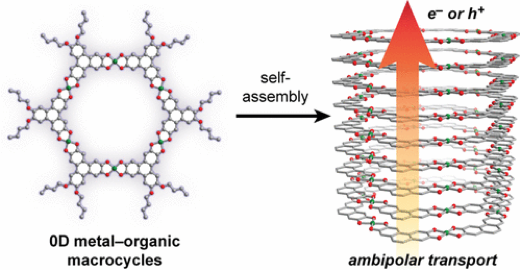
3.1.1 Conjugated Metal–Organic Macrocycles: Synthesis, Characterization, and Electrical Conductivity
Leo B. Zasada, Lorenzo Guio, Austin A. Kamin, Diwash Dhakal, Madison Monahan, Gerald T. Seidler, Christine K. Luscombe, Dianne J. Xiao,J. Am. Chem. Soc., 2022, 144, 4515-4521
The dimensional reduction of solids into smaller fragments provides a route to achieve new physical properties and gain deeper insight into the extended parent structures. Here, we report the synthesis of CuTOTP-OR (TOTPn– = 2,3,6,7-tetraoxidotriphenylene), a family of copper-based macrocycles that resemble truncated fragments of the conductive two-dimensional (2D) metal–organic framework Cu3(HHTP)2 (HHTP = 2,3,6,7,10,11-hexahydroxytriphenylene). The planar metal–organic macrocycles self-assemble into ordered nanotubes with internal diameters of 2 nm and short interlayer distances of 3.20 Å. Strong π–π stacking interactions between macrocycles facilitate out-of-plane charge transport, and pressed pellet conductivities as high as 2(1) × 10–3 S cm–1 are observed. Peripheral alkyl functionalization enhances solution processability and enables the fabrication of thin-film field-effect transistor devices. Ambipolar charge transport is observed, suggesting that similar behavior may be operative in Cu3(HHTP)2. By coupling the attractive features of metal–organic frameworks with greater processability, these macrocycles enable facile device integration and a more nuanced understanding of out-of-plane charge transport in 2D conductive metal–organic frameworks.
3.1.2. Circular Discovery in Small Molecule and Conjugated Polymer Synthetic Methodology
Amy L. Mayhugh, Preeti Yadav, Christine K. Luscombe, J. Am. Chem. Soc., 2022, 144, 6123-6135
Simple and efficient methods are a key consideration for small molecule and polymer syntheses. Direct arylation polymerization (DArP) is of increasing interest for preparing conjugated polymers as an effective approach compared to conventional cross-coupling polymerizations. As DArP sees broader utilization, advancements are needed to access materials with improved properties and different monomer structures and to improve the scalability of conjugated polymer synthesis. Presented herein are considerations for developing new methods of conjugated polymer synthesis from small molecule transformations, exploring how DArP has successfully used this approach, and presenting how emerging polymerization methodologies are developing similarly. While it is common to adapt small molecule methods to polymerizations, we demonstrate the ways in which information gained from studying polymerizations can inform and inspire greater advancements in small molecule transformations. This circular approach to organic synthetic method development underlines the value of collaboration between small molecule and polymer-based synthetic research groups.

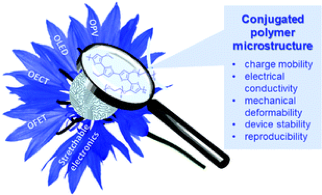
3.1.3. Gaining control over conjugated polymer morphology to improve the performance of organic electronics
Nadzeya A. Kukhta, Christine K. Luscombe, Chem. Commun, 2022, 58, 6982-6997. This article is part of the themed collections: 2022 Pioneering Investigators and Chemical Communications HOT Articles 2022. Also chosen as the front cover
Conjugated polymers (CPs) are widely used in various domains of organic electronics. However, the performance of organic electronic devices can be variable due to the lack of precise predictive control over the polymer microstructure. While the chemical structure of CPs is important, CP microstructure also plays an important role in determining the charge-transport, optical and mechanical properties suitable for a target device. Understanding the interplay between CP microstructure and the resulting properties, as well as predicting and targeting specific polymer morphologies, would allow current comprehension of organic electronic device performance to be improved and potentially enable more facile device optimization and fabrication. In this Feature Article, we highlight the importance of investigating CP microstructure, discuss previous developments in the field, and provide an overview of the key aspects of the CP microstructure-property relationship, carried out in our group over recent years.
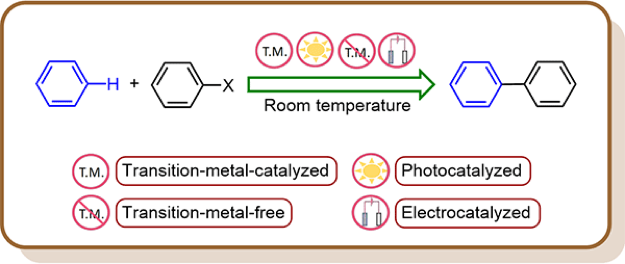
3.1.4. Recent Advances in Room-Temperature Direct C–H Arylation Methodologies
Preeti Yadav, Nivedha Velmurugan, Christine Luscombe, Synthesis, 2022, 55, 1-26.
In recent decades, direct C–H arylation has become a preferred tool for biaryl coupling over traditional cross-coupling methods owing to its operationally simple protocol, inherent atom and step economy, and reduced metallic waste. Several elegant methods have been developed that offer the facile transformation of usually inert Csp2–H bonds into Csp2–Csp2 bonds in a single synthetic operation. Despite many merits, a major drawback to this chemistry comes from the low reactivity of aryl C–H bonds, which often mandate harsh reaction conditions compromising sustainability. Hence, developing reaction protocols that require milder conditions has become an important goal in this area of research. This review article comprehensively highlights the synthesis and mechanistic aspects of direct C–H arylation reactions, which proceed at or below room temperature.
3.1.5. Synergistic catalysis for the synthesis of semiconducting polymers
Christine K. Luscombe, Samantha Phan, Isha Sanskriti, Polym. J., 2022, DOI: s41428-022-00719-8
Organic semiconductors have received much interest over the past few decades. As the field has progressed, so has the complexity of the molecular structures of organic semiconductors. Often, the highest-performing organic semiconductors (i.e., those with the highest charge mobility or those that provide the highest power conversion efficiencies in organic photovoltaics) involve complex syntheses, making them very challenging to synthesize, even by experienced synthetic chemists. In this focused review, we report on recent efforts in developing more efficient synthetic pathways. Specifically, the concept of synergistic catalysis, which involves the use of two or more catalysts with orthogonal reactivity to enable reactions that are not possible with the use of a single catalyst, is introduced. Synergistic catalysis allows for controlled polymerizations, room-temperature reactions, and/or polymerizations with greater regioselectivity, opening the door to more time-, labor-, cost-, and energy-saving methods for synthesizing semiconducting polymers.
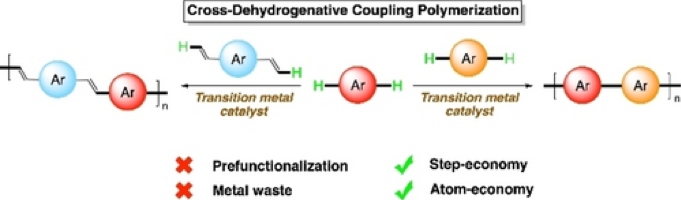
3.1.6. Cross-Dehydrogenative Coupling Polymerization via C–H Activation for the Synthesis of Conjugated Polymers
Baitan Chakraborty and Christine Luscombe, Angew. Chem. Int. Ed., 2023, in press. DOI: 10.1002/anie.202301247
Owing to their versatile (opto)electronic properties, conjugated polymers have found application in several organic electronic devices. Cross-coupling reactions such as Stille, Suzuki, Kumada couplings, and direct arylation reactions have proved to be effective for their synthesis. More atom-efficient oxidative direct arylation polymerization has also been reported for making homopolymers. However, growing interest toward donor-acceptor polymers has led to the recent emergence of cross-dehydrogenative coupling (CDC) polymerization to synthesize alternating copolymers without any prefunctionalization of monomers. Metal-catalyzed cross-coupling of two simple arenes via double C−H activation, or of an arene with an alkene via oxidative Heck-type reaction have been used so far for CDC polymerization. In this article, we discuss the development of CDC polymerization protocols along with the relevant small molecule CDC reactions for an improved understanding of these reactions.
3.2. Mixed ionic/electronic conducting polymers
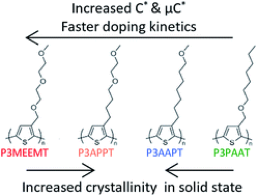
3.2.1. Impact of varying side chain structure on organic electrochemical transistor performance: a series of oligoethylene glycol-substituted polythiophenes
Shinya E. Chen, Lucas Q. Flagg, Jonathan W. Onorato, Lee J. Richter, J. Guo, Christine K. Luscombe, David S. Ginger, J. Mater. Chem. A, 2022, 10, 10738-10749
The electrochemical doping/dedoping kinetics, and the organic electrochemical transistor (OECT) performance of a series of polythiophene homopolymers with ethylene glycol units in their side chains using both kosmotropic and chaotropic anion solutions were studied. We compare their performance to a reference polymer, the polythiophene derivative with diethylene glycol side chains, poly(3-{[2-(2-methoxyethoxy)ethoxy]methyl}thiophene-2,5-diyl) (P3MEEMT). We find larger OECT material figure of merit, μC*, where μ is the carrier mobility and C* is the volumetric capacitance, and faster doping kinetics with more oxygen atoms on the side chains, and if the oxygen atom is farther from the polythiophene backbone. Replacing the oxygen atom close to the polythiophene backbone with an alkyl unit increases the film π-stacking crystallinity (higher electronic conductivity in the undoped film) but sacrifices the available doping sites (lower volumetric capacitance C* in OECT). We show that this variation in C* is the dominant factor in changing the μC* product for this family of polymers. With more oxygen atoms on the side chain, or with the oxygen atom farther from the polymer backbone, we observe both more passive swelling and higher C*. In addition, we show that, compared to the doping speed, the dedoping speed, as measured via spectroelectrochemistry, is both generally faster and less dependent on ion species or side chain oxygen content. Last, through OECT, electrochemical impedance spectroscopy (EIS) and spectroelectrochemistry measurements, we show that the chaotropic anion PF6− facilitates higher doping levels, faster doping kinetics, and lower doping thresholds compared to the kosmotropic anion Cl−, although the exact differences depend on the polymer side chains. Our results highlight the importance of balancing μ and C* when designing molecular structures for OECT active layers.
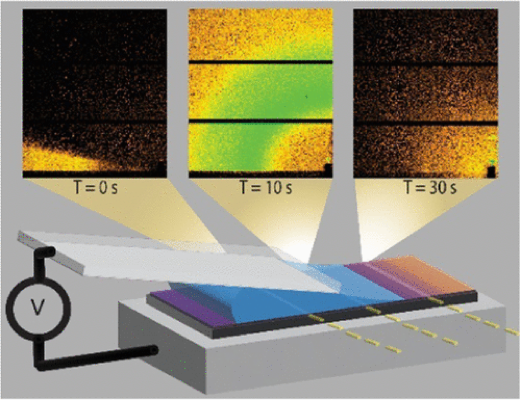
3.2.2. In Situ Studies of the Swelling by an Electrolyte in Electrochemical Doping of Ethylene Glycol-Substituted Polythiophene
Lucas Q. Flagg, Laurent E. Asselta, Nicholas D'Antona, Tommaso Nicolini, Natalie Stingelin, Jonathan W. Onorato, Christine K. Luscombe, Ruipeng Li, Lee J. Richter, ACS Appl. Mater. Interfaces, 2022, 14, 29052-29060.
Organic mixed ionic electronic conductors (OMIECs) have the potential to enable diverse new technologies, ranging from biosensors to flexible energy storage devices and neuromorphic computing platforms. However, a study of these materials in their operating state, which convolves both passive and potential-driven solvent, cation, and anion ingress, is extremely difficult, inhibiting rational material design. In this report, we present a novel approach to the in situ studies of the electrochemical switching of a prototypical OMIEC based on oligoethylene glycol (oEG) substitution of semicrystalline regioregular polythiophene via grazing-incidence X-ray scattering. By studying the crystal lattice both dry and in contact with the electrolyte while maintaining potential control, we can directly observe the evolution of the crystalline domains and their relationship to film performance in an electrochemically gated transistor. Despite the oEG side-chain enabling bulk electrolyte uptake, we find that the crystalline regions are relatively hydrophobic, exhibiting little (less than one water per thiophene) swelling of the undoped polymer, suggesting that the amorphous regions dominate the reported passive swelling behavior. With applied potential, we observe that the π–π separation in the crystals contracts while the lamella spacing increases in a balanced fashion, resulting in a negligible change in the crystal volume. The potential-induced changes in the crystal structure do not clearly correlate to the electrical performance of the film as an organic electrochemical transistor, suggesting that the transistor performance is strongly influenced by the amorphous regions of the film.
3.2. Polymers for stretchable applications
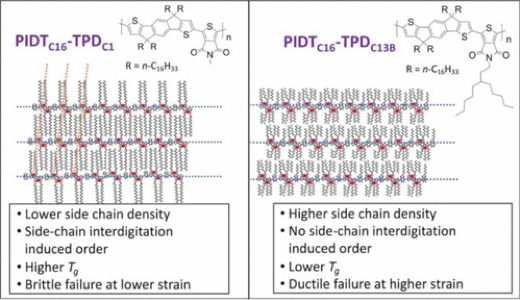
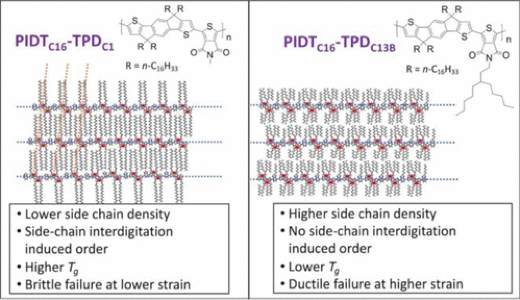
3.3.1. Influence of Side Chain Interdigitation on Strain and Charge Mobility of Planar Indacenodithiophene Copolymers
Parker J. W. Sommerville, Alex H. Balzer, Garrett Lecroy, Lorenzo Guio, Yunfei Wang, Jonathan W. Onorato, Nadzeya A. Kukhta, Xiaodan Gu, Alberto Salleo, Natalie Stingelin, and Christine K. Luscombe, ACS Polym. Au, 2022, DOI: 10.1021/acspolymersau.2c00034
Indacenodithiophene (IDT) copolymers are a class of conjugated polymers that have limited long-range order and high hole mobilities, which makes them promising candidates for use in deformable electronic devices. Key to their high hole mobilities is the coplanar monomer repeat units within the backbone. Poly(indacenodithiophene-benzothiadiazole) (PIDTC16-BT) and poly(indacenodithiophene-thiapyrollodione) (PIDTC16-TPDC1) are two IDT copolymers with planar backbones, but they are brittle at low molecular weight and have unsuitably high elastic moduli. Substitution of the hexadecane (C16) side chains of the IDT monomer with isocane (C20) side chains was performed to generate a new BT-containing IDT copolymer: PIDTC20-BT. Substitution of the methyl (C1) side chain on the TPD monomer for an octyl (C8) and 6-ethylundecane (C13B) afford two new TPD-containing IDT copolymers named PIDTC16-TPDC8 and PIDTC16-TPDC13B, respectively. Both PIDTC16-TPDC8 and PIDTC16-TPDC13B are relatively well deformable, have a low yield strain, and display significantly reduced elastic moduli. These mechanical properties manifest themselves because the lengthened side chains extending from the TPD-monomer inhibit precise intermolecular ordering. In PIDTC16-BT, PIDTC20-BT and PIDTC16-TPDC1 side chain ordering can occur because the side chains are only present on the IDT subunit, but this results in brittle thin films. In contrast, PIDTC16-TPDC8 and PIDTC16-TPDC13B have disordered side chains, which seems to lead to low hole mobilities. These results suggest that disrupting the interdigitation in IDT copolymers through comonomer side chain extension leads to more ductile thin films with lower elastic moduli, but decreased hole mobility because of altered local order in the respective thin films. Our work, thus, highlights the trade-off between molecular packing structure for deformable electronic materials and provides guidance for designing new conjugated polymers for stretchable electronics.
3.4. Microplastics in the environment
3.4.1. The effect of weathering environments on microplastic chemical identification with Raman and IR spectroscopy: Part I. polyethylene and polypropylene
Samantha Phan, Jacqueline L. Padilla-Gamiño, Christine K. Luscombe, Polym. Test., 2022, 116, 107752
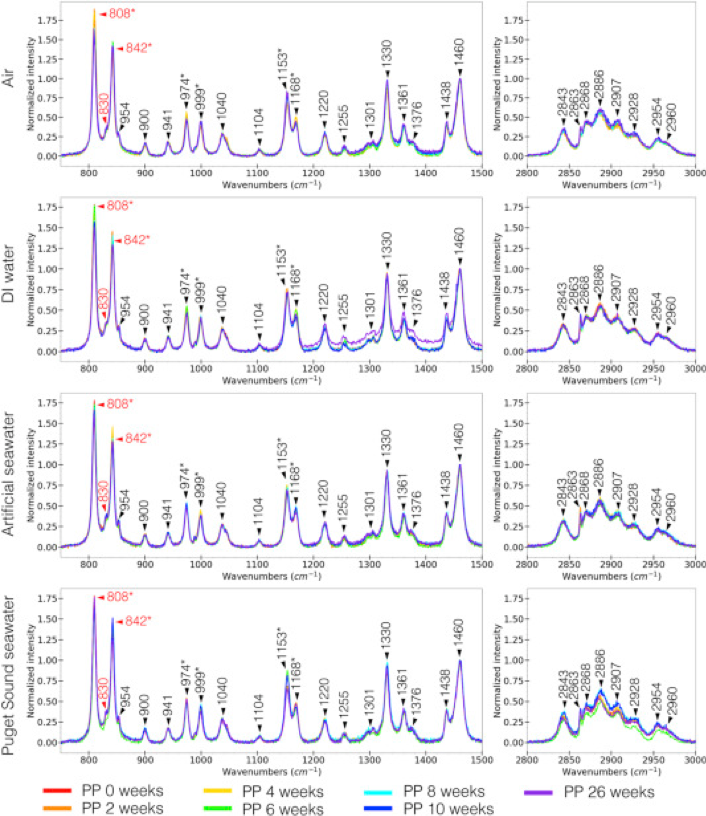
Chemical identification of microplastics is a crucial step in understanding the sources of microplastics and studying the effects of microplastic pollution. A major challenge to microplastics identification is that weathered microplastics undergo surface chemical changes that differ from their unweathered counterparts which hinders spectroscopic matching and thus identification. While IR spectroscopy is a common technique used for microplastics identification, Raman spectroscopy is a complementary technique and its higher spatial resolution capabilities (capable of analyzing particles as small as 1 μm) make it a popular tool to identify smaller microplastics that can otherwise go undetected by IR techniques. Currently, there is limited Raman spectroscopic information on weathered microplastics. Herein, we investigate the effects of artificial weathering on polyethylene and polypropylene microplastics for 0–26 weeks in four different weathering conditions (air, DI water, artificial seawater, and Puget Sound seawater) using Raman and IR spectroscopy. Microplastics weathering in different environments reveal that they can lead to different IR spectra, suggestive of varying degradation pathways for plastics, but relatively similar Raman spectra. Raman spectra, however, do show variations in peaks associated with the crystallinity and amorphous regions in the polymers which indicate morphological changes in the weathered microplastics. Overall, Raman spectroscopy acts as a practical interrelated method that can be used in lieu of IR spectroscopy in cases where weathering can complicate plastics identification by spectroscopic matching. This work aims to provide spectral information to facilitate marine microplastic chemical identification and encourage more investigations on the conditions that influence microplastic weathering.
3.4.2. Recent trends in marine microplastic modeling and machine learning tools: Potential for long-term microplastic monitoring
Samantha Phan and Christine K. Luscombe, J. Appl. Phys., 2023, 133, 020701. DOI: 10.1063/5.0126358
This paper was part of the Special Collection Recognizing Women in Applied Physics. Also highlighted as a Scilight.
The increase in the global demand for plastics, and more recently during the pandemic, is a major concern for the future of plastic waste pollution and microplastics. Efficient microplastic monitoring is imperative to understanding the long-term effects and progression of microplastic effects in the environment. Numerical models are valuable in studying microplastic transport as they can be used to examine the effects of different parameters systematically to help elucidate the fate and transport processes of microplastics, thus providing a holistic view of microplastics in the ocean environment. By incorporating physical parameters (such as size, shape, density, and identity of microplastics), numerical models have gained better understanding of the physics of microplastic transport, predicted sinking velocities more accurately, and estimated microplastic pathways in marine environments. However, availability of large amounts of information about microplastic physical and chemical parameters is sparse. Machine learning and computer-vision tools can aid in acquiring environmental information and provide input to develop more accurate models and verify their predictions. More accurate models can further the understanding of microplastic transport, facilitate monitoring efforts, and thus optimize where more data collection can take place to ultimately improve machine learning tools. This review offers a perspective on how image-based machine learning can be exploited to help uncover the physics of microplastic transport behaviors. Additionally, the authors hope the review inspires studies that can bridge the gap between numerical modeling and machine learning for microplastic analysis to exploit their joined potential.
3.5 IUPAC
3.5.1. A Brief Guide to Polymerization Terminology (IUPAC Technical Report)
Christine K. Luscombe, Graeme Moad, Roger C. Hiorns, Richard G. Jones, Daniel J. Keddie, John B. Matson, Jan Merna, Tamaki Nakano, Gregory T. Russell, Paul D. Topham, Pure Appl. Chem., 2022, 94, 1079-1084
The use of self-consistent terminology to describe polymerizations is important for litigation, patents, research and education. Imprecision in these areas can be both costly and confusing. To address this situation the International Union of Pure and Applied Chemistry (IUPAC) has made recommendations, which are summarized below. In the version shown as the supplementary material, references and hyperlinks lead to source documents; screen tips contain definitions published in IUPAC recommendations. More details can also be found in the IUPAC Purple Book. This guide is one of a series on terminology and nomenclature. Refer to the supplementary material for the complete and interactive version of this brief guide.
3.5.2. Reconsidering terms for mechanisms of polymer growth: the “step-growth” and “chain-growth” dilemma
Chin Han Chan, Jiun-Tai Chen, Wesley S. Farrell, Christopher M. Fellows, Daniel J. Keddie, Christine K. Luscombe, John B. Matson, Jan Merna, Graeme Moad, Gregory T. Russell, Patrick Théato, Paul D. Topham, and Lydia Sosa Vargas,Polym. Chem., 2022, 13, 2262-2270.
The terms “step-growth polymerization” and “chain-growth polymerization” are used widely in both written and oral communications to describe the two main mechanisms of polymer growth. As members of the Subcommittee on Polymer Terminology (SPT) in the Polymer Division of the International Union of Pure and Applied Chemistry (IUPAC), we are concerned that these terms are confusing because they do not describe the fundamental differences in the growth of polymers by these methods. For example, both polymerization methods are comprised of a series of steps, and both produce polymer chains. In an effort to recommend comprehensive terms, a 1994 IUPAC Recommendation from the then version of SPT suggested polycondensation and polyaddition as terms for the two variants of “step-growth polymerization”, and similarly chain polymerization and condensative chain polymerization for the two variants of “chain-growth polymerization”. However, these terms also have shortcomings. Adding to the confusion, we have identified a wide variety of other terms that are used in textbooks for describing these basic methods of synthesizing polymers from monomers. Beyond these issues with “step-growth” and “chain-growth”, synthesis of polymers one monomer unit at a time presents a related dilemma in that this synthetic strategy is wholly encompassed by neither of the traditional growth mechanisms. One component of the mission of IUPAC is to develop tools for the clear communication of chemical knowledge around the world, of which recommending definitions for terms is an important element. Here we do not endorse specific terms or recommend new ones; instead, we aim to convey our concerns with the basic terms typically used for classifying methods of polymer synthesis, and in this context we welcome dialogue from the broader polymer community in a bid to resolve these issues.
3.5.3. Terminology for chain polymerization (IUPAC Recommendations 2021)
Christopher M. Fellows, Richard G. Jones, Daniel J. Keddie, Christine K. Luscombe, John B. Matson, Krzystof Matyjaszewski, Jan Merna, Graeme Moad, Tamaki Nakano, Stanislaw Penczek, Gregory T. Russell, and Paul D. Topham, Pure Appl. Chem., 2022, 94, 1093-1147.
Chain polymerizations are defined as chain reactions where the propagation steps occur by reaction between monomer(s) and active site(s) on the polymer chains with regeneration of the active site(s) at each step. Many forms of chain polymerization can be distinguished according to the mechanism of the propagation step (e.g., cyclopolymerization – when rings are formed, condensative chain polymerization – when propagation is a condensation reaction, group-transfer polymerization, polyinsertion, ring-opening polymerization – when rings are opened), whether they involve a termination step or not (e.g., living polymerization – when termination is absent, reversible-deactivation polymerization), whether a transfer step is involved (e.g., degenerative-transfer polymerization), and the type of chain carrier or active site (e.g., radical, ion, electrophile, nucleophile, coordination complex). The objective of this document is to provide a language for describing chain polymerizations that is both readily understandable and self-consistent, and which covers recent developments in this rapidly evolving field.
3.6 Collaborative work
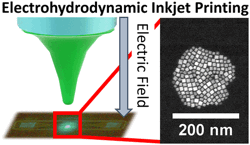
3.6.1. Direct Patterning of Perovskite Nanocrystals on Nanophotonic Cavities with Electrohydrodynamic Inkjet Printing
Theodore A. Cohen, David Sharp, Kyle T. Kluherz, Yueyang Chen, Christopher Munley, Rayne T. Anderson, Connor J. Swanson, James J. De Yoreo, Christine K. Luscombe, Arka Majumdar, Daniel R. Gamelin, J. Devin Mackenzie, Nano Lett., 2022, 14, 5681-5688
Overcoming the challenges of patterning luminescent materials will unlock additive and more sustainable paths for the manufacturing of next-generation on-chip photonic devices. Electrohydrodynamic (EHD) inkjet printing is a promising method for deterministically placing emitters on these photonic devices. However, the use of this technique to pattern luminescent lead halide perovskite nanocrystals (NCs), notable for their defect tolerance and impressive optical and spin coherence properties, for integration with optoelectronic devices remains unexplored. In this work, we additively deposit nanoscale CsPbBr3 NC features on photonic structures via EHD inkjet printing. We perform transmission electron microscopy of EHD inkjet printed NCs to demonstrate that the NCs’ structural integrity is maintained throughout the printing process. Finally, NCs are deposited with sub-micrometer control on an array of parallel silicon nitride nanophotonic cavities and demonstrate cavity–emitter coupling via photoluminescence spectroscopy. These results demonstrate EHD inkjet printing as a scalable, precise method to pattern luminescent nanomaterials for photonic applications.
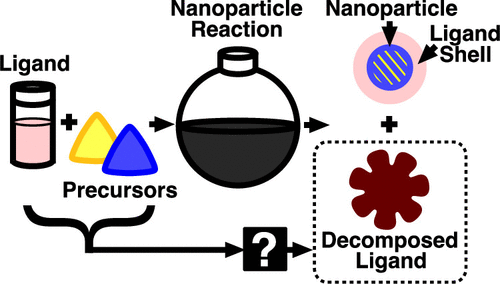
3.6.2. Ligand Decomposition during Nanoparticle Synthesis: Influence of Ligand Structure and Precursor Selection
Breena M. Sperry, Nadzeya A. Kukhta, Yunping Huang, and Christine K. Luscombe Chem. Mater., 2023, 35, 570-583.
Aliphatic amine and carboxylic acid ligands are widely used as organic solvents during the bottom-up synthesis of inorganic nanoparticles (NPs). Although the ligands’ ability to alter final NP properties has been widely studied, side reactivity of these ligands is emerging as an important mechanism to consider. In this work, we study the thermal decomposition of common ligands with varying functional groups (amines and carboxylic acids) and bond saturations (from saturated to polyunsaturated). Here, we investigate how these ligand properties influence decomposition in the absence and presence of precursors used in NP synthesis. We show that during the synthesis of inorganic chalcogenide NPs (Cu2ZnSnS4, CuxS, and SnSx) with metal acetylacetonate precursors and elemental sulfur, the ligand pyrolyzes, producing alkylated graphitic species. Additionally, there was less to no ligand decomposition observed during the sulfur-free synthesis of ZnO and CuO with metal acetylacetonate precursors. These results will help guide ligand selection for NP syntheses and improve reaction purity, an important factor in many applications.
4. Publications
4.1 Journals
Peer-reviewed publications
- Leo B. Zasada, Lorenzo Guio, Austin A. Kamin, Diwash Dhakal, Madison Monahan, Gerald T. Seidler, Christine K. Luscombe, Dianne J. Xiao “Conjugated Metal–Organic Macrocycles: Synthesis, Characterization, and Electrical Conductivity” J. Am. Chem. Soc., 2022, 144, 4515-4521. DOI: 10.1021/jacs.1c12596
- Amy L. Mayhugh, Preeti Yadav, Christine K. Luscombe “Circular Discovery in Small Molecule and Conjugated Polymer Synthetic Methodology” J. Am. Chem. Soc., 2022, 144, 6123-6135. DOI: 10.1021/jacs.1c12455
- Shinya E. Chen, Lucas Q. Flagg, Jonathan W. Onorato, Lee J. Richter, J. Guo, Christine K. Luscombe, David S. Ginger “Impact of varying side chain structure on organic electrochemical transistor performance: a series of oligoethylene glycol-substituted polythiophenes” J. Mater. Chem. A, 2022, 10, 10738-10749. DOI: 10.1039/D2TA00683A
- Nadzeya A. Kukhta, Christine K. Luscombe “Gaining control over conjugated polymer morphology to improve the performance of organic electronics” Chem. Commun, 2022, 58, 6982-6997. DOI: 10.1039/D2CC01430K. This article is part of the themed collections: 2022 Pioneering Investigators and Chemical Communications HOT Articles 2022. Also chosen as the front cover.
- Lucas Q. Flagg, Laurent E. Asselta, Nicholas D'Antona, Tommaso Nicolini, Natalie Stingelin, Jonathan W. Onorato, Christine K. Luscombe, Ruipeng Li, Lee J. Richter “In Situ Studies of the Swelling by an Electrolyte in Electrochemical Doping of Ethylene Glycol-Substituted Polythiophene” ACS Appl. Mater. Interfaces, 2022, 14, 29052-29060. DOI: 10.1021/acsami.2c06169
- Theodore A. Cohen, David Sharp, Kyle T. Kluherz, Yueyang Chen, Christopher Munley, Rayne T. Anderson, Connor J. Swanson, James J. De Yoreo, Christine K. Luscombe, Arka Majumdar, Daniel R. Gamelin, J. Devin Mackenzie “Direct Patterning of Perovskite Nanocrystals on Nanophotonic Cavities with Electrohydrodynamic Inkjet Printing” Nano Lett., 2022, 14, 5681-5688. DOI: 10.1515/pac-2021-0115
- Christine K. Luscombe, Graeme Moad, Roger C. Hiorns, Richard G. Jones, Daniel J. Keddie, John B. Matson, Jan Merna, Tamaki Nakano, Gregory T. Russell, Paul D. Topham “A brief guide to polymerization terminology (IUPAC Technical Report)” Pure Appl. Chem., 2022, 94, 1079-1084. DOI: 10.1515/pac-2021-0115
- Preeti Yadav, Nivedha Velmurugan, Christine Luscombe “Recent Advances in Room-Temperature Direct C-H Arylation Methodologies” Synthesis, 2022, 55, 1-26. DOI: 10.1055/a-1939-7052
- Samantha Phan, Jacqueline L. Padilla-Gamiño, Christine K. Luscombe “The effect of weathering environments on microplastic chemical identification with Raman and IR spectroscopy: Part I. polyethylene and polypropylene” Polym. Test., 2022, 116, 107752. DOI: 10.1016/j.polymertesting.2022.107752
- Parker J. W. Sommerville, Alex H. Balzer, Garrett Lecroy, Lorenzo Guio, Yunfei Wang, Jonathan W. Onorato, Nadzeya A. Kukhta, Xiaodan Gu, Alberto Salleo, Natalie Stingelin, and Christine K. Luscombe “Influence of Side Chain Interdigitation on Strain and Charge Mobility of Planar Indacenodithiophene Copolymers” ACS Polym. Au, 2022, DOI: 10.1021/acspolymersau.2c00034
- Christine K. Luscombe, Samantha Phan, Isha Sanskriti “Synergistic catalysis for the synthesis of semiconducting polymers” Polym. J., 2022, DOI: s41428-022-00719-8
- Chin Han Chan, Jiun-Tai Chen, Wesley S. Farrell, Christopher M. Fellows, Daniel J. Keddie, Christine K. Luscombe, John B. Matson, Jan Merna, Graeme Moad, Gregory T. Russell, Patrick Théato, Paul D. Topham, and Lydia Sosa Vargas “Reconsidering terms for mechanisms of polymer growth: the “step-growth” and “chain-growth” dilemma” Polym. Chem., 2022, 13, 2262-2270. DOI: 10.1039/D2PY00086E
- Christopher M. Fellows, Richard G. Jones, Daniel J. Keddie, Christine K. Luscombe, John B. Matson, Krzystof Matyjaszewski, Jan Merna, Graeme Moad, Tamaki Nakano, Stanislaw Penczek, Gregory T. Russell, and Paul D. Topham “Terminology for chain polymerization (IUPAC Recommendations 2021)” Pure Appl. Chem., 2022, 94, 1093-1147. DOI: 10.1515/pac-2020-1211
- Breena M. Sperry, Nadzeya A. Kukhta, Yunping Huang, and Christine K. Luscombe “Ligand Decomposition during Nanoparticle Synthesis: Influence of Ligand Structure and Precursor Selection” Chem. Mater., 2023, 35, 570-583. DOI: 10.1021/acs.chemmater.2c03006
- Samantha Phan and Christine K. Luscombe “Recent trends in marine microplastic modeling and machine learning tools: Potential for long-term microplastic monitoring” J. Appl. Phys., 2023, 133, 020701. DOI: 10.1063/5.0126358. This paper was part of the Special Collection Recognizing Women in Applied Physics. Also highlighted as a Scilight.
- Baitan Chakraborty and Christine Luscombe “Cross-Dehydrogenative Coupling Polymerization via C–H Activation for the Synthesis of Conjugated Polymers” Angew. Chem. Int. Ed., 2023, in press. DOI: 10.1002/anie.202301247
Non-peer-reviewed publications
- Diane Purchase, Annemieke Farenhorst, Hemda Garelick, Nadia G Kandile, Christine Luscombe, Laura McConnell, Bulent Mertoglu, Bradley Miller, Fani Sakellariadou, Roberto Terzano, Weiping Wu “Environmental Chemistry and Sustainability” Chem. Int., 2022, 44, 45-48. DOI: 10.1515/ci-2022-0231
- Christine K. Luscombe “In memoriam Melissa Chan 1974–2022” Pure Appl. Chem., 2023, 95, 79-80. DOI: 10.1515/pac-2023-2001
- Isha Sanskriti, Aditya Singh, Christine K. Luscombe “Engineered hierarchically ordered structures in conducting polymers enabling enhanced performance of lithium-ion batteries” Joule, 2023, 7, 465-468. DOI: 10.1016/j.joule.2023.02.019
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
Contributed conference presentations
- Liwen Xingand Christine K. Luscombe, Synergistic dual transition metal catalysis for the synthesis of semiconducting polymers, Society of Polymer Science Japan Annual meeting, May 2022, Virtual
Invited symposium and conference presentations
- Christine K. Luscombe, Side chain engineering control of mixed conduction in oligoethylene glycol-substituted polythiophenes, SPIE Photonics Europe, April 2022, Virtual
- Christine K. Luscombe, Development in semiconducting polymer synthesis, Poly-Char 2022, May 2022, Virtual
- Christine K. Luscombe, Side chain engineering control of mixed conduction in oligoethylene glycol-substituted polythiophene, Symposium in honor of Prof. Seth Marder, May 2022, UC Boulder, Colorado, USA.
- Christine K. Luscombe, Side-Chain Engineering to Balance Ionic and Electronic Conductivities in Mixed Ionic/Electronic Conductors, MRS National Spring Meeting 2022, May 2022, Virtual
- Christine K. Luscombe, Synergistic dual transition metal catalysis for the synthesis of semiconducting polymers, Bordeaux Polymer Conference, June 2022, Virtual
- Christine K. Luscombeand Yunping Huang, Organic dyes derived from molecules in cacao beans for use in lighting applications, 242ndECS Meeting, June 2022, Vancouver, Canada
- Christine K. Luscombe, The future of organic electronics and plastics, OIST-Keio Showcase Talk Series 3, June 2022, Virtual.
- Christine K. Luscombe, Synergistic dual transition metal catalysis for the synthesis of semiconducting polymers, 2022 World Polymer Congress, July 2022, Winnipeg, Canada
- Christine K. Luscombe, Synergistic dual transition metal catalysis for the synthesis of semiconducting polymers, American Chemical Society Spring National Meeting, August 2022, Chicago, USA
- Christine K. Luscombe, Side chain engineering control of mixed conduction in oligoethylene glycol-substituted polythiophenes, Society of Polymer Science Japan Symposium on Macromolecules, September 2022, Sapporo, Japan
- Christine K. Luscombe, Towards a more efficient synthesis of semiconducting polymers,2022 International Symposium on Natural Sciences, Incheon National University, October 2022, Virtual
- Christine K. Luscombe, Side-chain variation in polythiophene derivatives to affect mixed ionic/electronic conductor performance, Telluride Workshop on Organic Mixed Conductors, October 2022, Telluride, CO, USA
- Christine K. Luscombe and Liwen Xing, Synergistic dual transition metal catalysis for the synthesis of semiconducting polymers, Japan-US Workshop on Organic/Inorganic Hybrid Materials, November 2022, Mishima, Japan
- Christine K. Luscombe and Liwen Xing, Synergistic dual transition metal catalysis for the synthesis of semiconducting polymers, The 15thJapan-Belgium Symposium on Polymer Science, November 2022, Mishima, Japan
- Christine K. Luscombe, Efficient syntheses of semiconducting polymers, The 2nd Workshop on Ladder Polymer Science, November 2022, OIST, Japan
- Christine K. Luscombe, Synergistic catalysis for the synthesis of semiconducting polymers, Sustainable energy materials and technologies for a low carbon future, March 2023, KAUST, Saudi Arabia
Plenary/Keynote speaker
- Christine K. Luscombe, Side-Chain Engineering of mixed ionic/electronic conductors, Australasian Community for Advanced Organic Semiconductors (AUCAOS) 2022, December 2022, Tweed Heads, Australia
- Christine K. Luscombe, Sustainable syntheses of semiconducting polymers, The 17thPacific Polymer Conference, December 2022, Brisbane, Australia
Seminars
- Christine K. Luscombe, Side-Chain Engineering to Balance Ionic and Electronic Conductivities in Mixed Ionic/Electronic Conductors, University of Kyoto, Institute for Integrated Cell-Material Sciences (iCeMS), October 2022
- Christine K. Luscombe, Synergistic catalysis for the synthesis of semiconducting polymers, University of Kyoto, Graduate School of Engineering, October 2022
- Christine K. Luscombe, Semiconducting polymers: New horizons and unmet challenges, University of Oregon, Department of Chemistry, November 2022, virtual
- Christine K. Luscombe, Synergistic catalysis towards the development of efficient pathways to synthesize semiconducting polymers, University of Victoria, Department of Chemistry, Canada, January 2023, virtual
Poster presentation
- Samantha Phan, Isha Sanskriti, Preeti Yadav, Lorenzo Guio, Aditya Singh, Christine Luscombe, A comparison of global DEI strategies and their effectiveness, OIST Inclusive Leadership Symposium, February 2023, OIST, Japan.
- Chin Han Chan, Jiun-Tai Chen, Wesley S. Farrell, Christopher M. Fellows, Daniel J. Keddie, Christine K. Luscombe, John B. Matson, Jan Merna, Graeme Moad, Gregory T. Russell, Patrick Théato, Paul D. Topham, Lydia Sosa Vargas, The “step-growth” and “chain-growth” dilemma: why we need to reconsider the terminology used, #RSCPoster Global Twitter Poster Competition 2023, virtual
- Lorenzo Guio, Christine K. Luscombe, Making conducting polymers greener: solvent-free mechanochemical synthesis of D-A copolymers, Sustainable energy materials and technologies for a low carbon future, March 2023, KAUST, Saudi Arabia
5. Other Specific Achievements
- Christine Luscombe (PI), Kakenhi Grant-in Aid for Research Activity, September 2022-August 2023, ¥2,200,000, 22K20547, Synergistic catalysis for the sustainable synthesis of semiconducting polymers
- Christine Luscombe (PI), SHINKA, ¥1,000,000, April 2022-March 2023, Advanced functional polymer/carbon composites with three-dimensionally tailored nanostructures
- Preeti Yadav (PI), SHINKA, ¥800,000, September 2022-March 2023, Presence and Identity of Microplastics within and around Marine Research Stations
- Christine Luscombe (co-author), 1stprize for Materials for Global Twitter Poster Conference organized by the Royal Society of Chemistry, https://www.rsc.org/our-events/rsc-poster/winners/#RSCMat
- Christine Luscombe, SPSJ Science Award 2022 from the Society of Polymer Science Japan
6. Meetings and Events
6.1. Seminar "Is π-single bonding possible?" by Prof. Manabu Abe
- Date: September 22nd, 2022
- Venue: C209, Center Bldg
- Speaker: Manabu Abe, Ph.D. (Professor, Hiroshima University)
6.2. Seminar "Why does diversity matter in a research institution? From my personal experiences at RIKEN" by Dr. Yuko Harayama
-
Date: October 12th, 2022
- Venue: B250, Center Bldg / Zoom
- Speaker: Dr. Yuko Harayama, Professor Emeritus, Tohoku University. Target audience: Everyone at OIST.
6.3. 2023 IUPAC Global Women's Breakfast at OIST
- Date: February 14th, 2023
- Venue: OIST Conference Center, Meeting Room 1
- The aim of the GWB (Global Women’s Breakfast) series is to celebrate the accomplishments of Women in Science and to inspire younger generations to pursue careers in science. This year at OIST we will celebrate with a breakfast/workshop and host keynote speaker Prof. Yukiko Goda! https://www.oist.jp/news-center/news/2023/3/7/what-we-can-do-now-break-down-invisible-gender-barriers
6.4. Seminar"Polymer-Based Organic Electrochemical Transistors: Neuromorphic and Biosensor Applications" by Prof. Shunsuke Yamamoto
- Date: March 13th, 2023
- Venue: L4E48
- Speaker: Shunsuke Yamamoto, Ph.D., Assistant Professor, Graduate School of Engineering, Tohoku University.
7. Others
7.1. Keio Summer Camp
7.2. Okinawa Konwakai
Christine Luscombe was a speaker at the event, January 2023
7.3. Online lecture to Fukushima high school students
Christine Luscombe spoke about her research at this online event, February 2023
7.4. OIST Science Challenge 2023
- Lorenzo Guio and Baitan Chakraborty led a lab tour, March 2023
- Callum Hudson and Samatha Phan led a science activity on Detection and characterization of microplastics in the environment, March 2023

7.5. C-Hub Inclusive Leadership Symposium 2023
Christine Luscombe served as Steering Committee member
7.6. IUPAC
Christine Luscombe serving as IUPAC Polymer Division President
7.7. Society of Polymer Science Japan
Christine Luscombe serving as Board member for SPSJ
7.8. ACS Macromolecules
Christine Luscombe serving as Associate Editor for Macromolecules
7.9. Faculty Council
Christine Luscombe serving as Chair of the Faculty Council
7.10. Editorial Advisory Boards
Christine Luscombe serving on the Advisory Boards for the following journals: Polymer Chemistry, Chemical Reviews, Synthetic Metals, ACS Applied Polymer Materials, Polymer Journal, Advanced Functional Materials, Annual Reviews of Materials Research, ACS Applied Materials and Interfaces, Polymer International, Advanced Electronic Materials
Serving as the Chair of the Advisory Board of Journal of Applied Physics



