FY2021 Annual Report
Optical Neuroimaging Unit
Professor Bernd Kuhn

(from left to right, front) Manami Gima, Anaí Echeverría, Lina Koronfel, Thato Mokhothu, Hoang-Dai Tran, Mohamed "Tabbal" Eltabbal, Bernd Kuhn, Sigita Augustinaite, Soumen Jana
Abstract
In the FY 2021 the Optical Neuroimaging Unit continued the projects of the previous years. Research projects focused on imaging neuronal activity in the brain of awake mice.
1. Staff
- Dr. Bernd Kuhn, Professor
- Dr. Christopher J. Roome, Staff Scientist
- Dr. Melody Lee, Postdoc
- Dr. Sigita Augustinaite, Technician
- Dr. Shinobu Nomura, Technician
- Dr. Mykola "Nik" Medvidov, Technician
- Dr. Kazuo Mori, Technician
- Soumen Jana, Ph.D. student
- Lina Koronfel, Ph.D. student
- Mohamed Mostafa Eltabbal, PhD student
- Isabel Anai Echeverria Oviedo. PhD student
- Cedric Galetzka, PhD student
- Miles Desforges, PhD student (co-supervised by Kenji Doya)
- Charlotte Denman, PhD student (Junghyun Jo Team)
- Thato Mokhothu, PhD student (co-supervised by Kazumasa Tanaka)
- Gaston Sivori, PhD student (so-supervised by Tomoki Fukai)
- Aleksandra Gavrilova, PhD student
- Qiyi Qian, Intern student
- Nina Harano, Intern student
- Manami Gima, Research Unit Administrator
Stem Cell and Organoid Research Group
- Dr. Junghyun Jo, Staff Scientist
- Dr. Hoang-Dai Tran, Postdoctoral Scholar
- Runrun Han, Research Technician
- Charlotte Denman, Ph.D. Student
- Dr. Min-Kyoung Shin, Visiting Researcher from CHA University, Korea
2. Collaborations
2.1 High-resolution imaging of barrel cortex through VSD and LFP recordings
- Description: Combining high-resolution electical recording with electrode arrays and voltage imaging
- Type of collaboration: Joint research
- Researchers:
- Associate Professor Stefano Vassanelli, Padua University, Italy
2.2 Motion correction of in vivo 2P imaging data
- Description: Development of algorythms for motion correction of 2P imaging data
- Type of collaboration: Joint research
- Researchers:
- Philipp Flotho, MSc., Systems Neuroscience & Neurotechnology Unit, Saarland University, Germany
2.3 Development of a genetic targeting mechanism for the pure electrochromic voltage-sensitive dye ANNINE-6
- Description: Synthesis of new voltage-sensitive dyes and in vivo testing of labeling
- Type of collaboration: Joint research
- Researchers:
- Dr. Eugene Khaskin, Science and Technology Group, OIST
2.4 In vitro voltage imaging of subthreshold activity in inferior olive neurons with ANNINE-6plus
- Description: Development of a new labeling technique for the voltage-sensitive dye ANNINE-6plus
- Type of collaboration: Joint research
- Researchers:
- Ass.Prof. Marylka Yoe Uusisaari
- Dr. Kevin Dorgans
2.5 Development of 2D and 3D human pluripotent stem cell differentiation platform for human disease modeling
- Description: Development of 2D and 3D human pluripotent stem cell differentiation platform for human disease modeling
- Researcher:
- Professor Dong Ryul Lee, , CHA University, Korea.
- Dr. Min-Kyoung Shin, CHA University, Korea.
2.6 Anoxia-Induced LTP at Hippocampal CA1 Synapses
- Description: Imaging astrocytes in vitro during and after anoxia
- Researcher:
- Professor Tomoyuki Takahashi, OIST
- Dr. Han-Ying Wang, OIST
2.7 Effect of the human FoxP2 gene on a licking task in mouse
- Description: Calcium imaging during a licking task in transgenc mice
- Researcher:
- Adjunct Professor Svante Pääbo, OIST
- Dr. Xiang-Chun Ju, OIST
3. Activities and Findings
3.1 Voltage Imaging
The Kuhn Unit continues to improve voltage imaging in cerebellar and cerebral cortex.
3.2 Ca2+ imaging in cortical layer 6
The Kuhn Unit continues to study neuronal Ca2+ activity in cortex.
3.3 Imaging of intrinsic optical signals
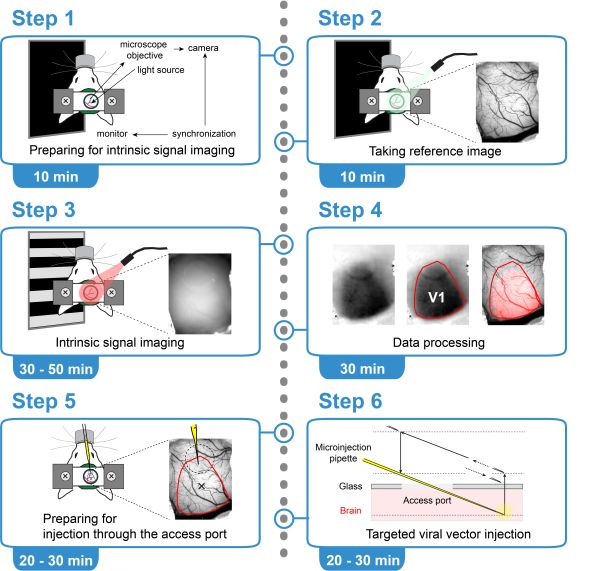
Intrinsic optical signals allow to map sensory cortical areas. We published a protocol which we used for maping the visual cortex of mice.
Intrinsic optical signal imaging (ISI) is a hemodynamic response-based technique to map the functional architecture of the cortex. ISI is often used as an auxiliary method to localize cortical areas for targeted electrophysiology, pharmacology, or imaging experiments. Here, we provide a protocol for ISI through a cranial window with an access port to identify the area of the primary visual cortex (V1) in a head-fixed mouse, followed by targeted viral vector injection, which enables subsequent two-photon imaging of V1 layer 6 corticothalamic neurons.
3.3 Imaging PKA activity in vivo
In the CNS, protein kinase A (PKA) is controlled by neuromodulators through G-protein-coupled receptors, therefore it is thought to be involved in multiple brain functions. However, PKA activity has not been observed with cellular or subcellular resolution in vivo.
In this study we imaged PKA activity of cortical neurons in awake mice. A genetically encoded single GFP-based PKA sensor, GakdYmut, was expressed after adeno-associated viral gene transfer. Mice were head fixed on a cylindrical treadmill and were allowed to walk ad libitum during imaging using two-photon microscopy through a chronic cranial window.
In somatosensory cortex, PKA activity rose in dendrites and somata of neurons in layer II/III and V with the onset of locomotion, and then reached a maximal amplitude after walking offset. PKA activity in dendrites and somata peaked 20 ± 9 seconds (n = 685) and 20± 6 seconds (n = 372), respectively, after the offset of locomotion, with maximal amplitudes of ΔF/F = 14%. PKA activity from labeled dendrites (43%) and somata (22%) showed synchronous responses greater than 2.5% ΔF/F (20s time window), in the same field of view. Similar PKA activity patterns were detected in the posterior parietal cortex, but not in the anterior cingulate cortex. Simultaneous imaging of PKA and calcium with the red calcium indicator protein rGECO revealed that PKA activation is independent of calcium influx due to spiking or bursting. We will try to identify neuromodulators which affect locomotion-related PKA activity in cortical neurons using pharmacology and electrophysiology.
3.4 In vitro voltage imaging of subthreshold activity in inferior olive neurons with ANNINE-6plus
Since the 1970s, a multitude of fluorescence-based methods for observing changes in neuronal membrane voltage have been developed, progressively improving in terms of signal sensitivity, speed, and targeting. While significant development has been seen in the past decade in engineering novel variants of genetically encoded voltage indicators (GEVI), synthetic dyes can, in many cases, be more straightforward and flexible tools to be used. Here, we describe in detail an improved dye-labeling method where the synthetic, pure electrochromic voltage-sensitive dye ANNINE-6plus is introduced into the brain region of interest via stereotactical injection in a mouse. Specifically, we have optimized the labeling protocol for effective voltage imaging of subthreshold activity in inferior olive. The homogenous labeling and excellent signal-to-noise ratio allow this method to reveal olivary subthreshold oscillations in high spatiotemporal resolution. In addition to the procedures related to dye introduction and optical imaging, we give a detailed description of the process of acute slice preparation from the mouse inferior olive.
3.5 Simultaneous two-photon voltage or calcium imaging and multi-channel local field potential recordings in barrel cortex of awake and anesthetized mice
Neuronal population activity, both spontaneous and sensory-evoked, generates propagating waves in cortex. However, high spatiotemporal-resolution mapping of these waves is difficult as calcium imaging, the work horse of current imaging, does not reveal subthreshold activity. Here, we present a platform combining voltage or calcium two-photon imaging with multi-channel local field potential (LFP) recordings in different layers of the barrel cortex from anesthetized and awake head-restrained mice. A chronic cranial window with access port allows injecting a viral vector expressing GCaMP6f or the voltage-sensitive dye (VSD) ANNINE-6plus, as well as entering the brain with a multi-channel neural probe. We present both average spontaneous activity and average evoked signals in response to multi-whisker air-puff stimulations. Time domain analysis shows the dependence of the evoked responses on the cortical layer and on the state of the animal, here separated into anesthetized, awake but resting, and running. The simultaneous data acquisition allows to compare the average membrane depolarization measured with ANNINE-6plus with the amplitude and shape of the LFP recordings. The calcium imaging data connects these data sets to the large existing database of this important second messenger. Interestingly, in the calcium imaging data, we found a few cells which showed a decrease in calcium concentration in response to vibrissa stimulation in awake mice. This system offers a multimodal technique to study the spatiotemporal dynamics of neuronal signals through a 3D architecture in vivo. It will provide novel insights on sensory coding, closing the gap between electrical and optical recordings.
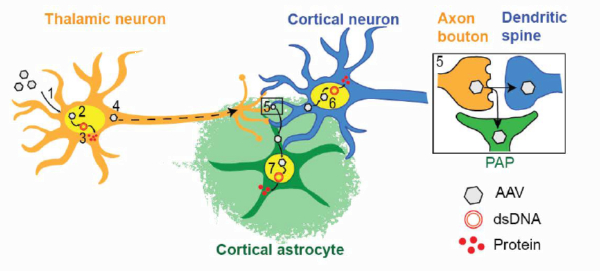
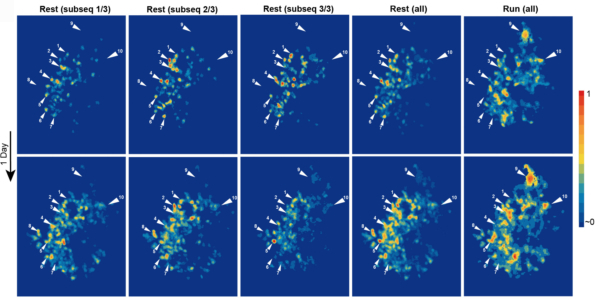
3.6 Ca2+ activity maps of astrocytes tagged by axoastrocytic AAV transfer
Astrocytes exhibit localized Ca2+ microdomain (MD) activity thought to be actively involved in information processing in the brain. However, functional organization of Ca2+ MDs in space and time in relationship to behavior and neuronal activity is poorly understood. Here, we first show that adeno-associated virus (AAV) particles transfer anterogradely from axons to astrocytes. Then, we use this axoastrocytic AAV transfer to express genetically encoded Ca2+ indicators at high-contrast circuit specifically. In combination with two-photon microscopy and unbiased, event-based analysis, we investigated cortical astrocytes embedded in the vibrissal thalamocortical circuit. We found a wide range of Ca2+ MD signals, some of which were ultrafast (≤300 ms). Frequency and size of signals were extensively increased by locomotion but only subtly with sensory stimulation. The overlay of these signals resulted in behavior-dependent maps with characteristic Ca2+ activity hotspots, maybe representing memory engrams. These functional subdomains are stable over days, suggesting subcellular specialization.
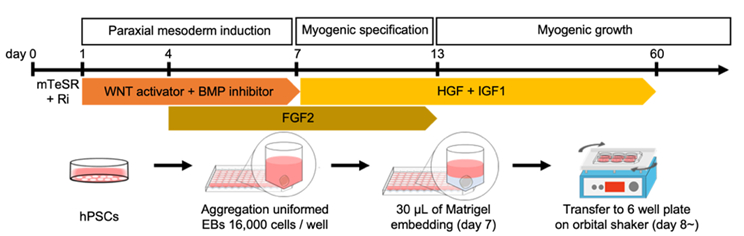
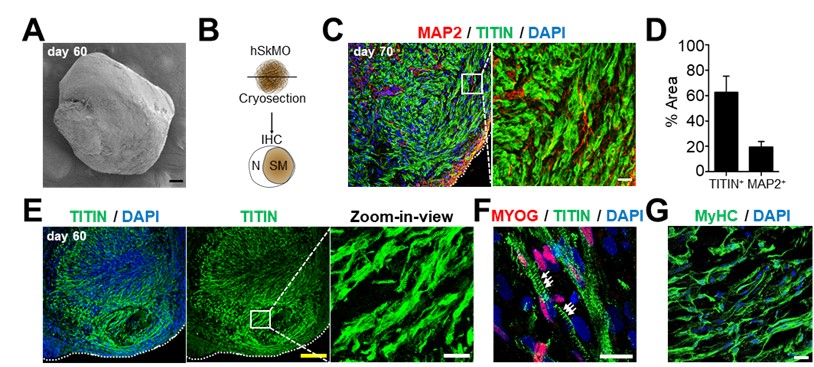
3.7 Establishment of a new method to create skeletal muscle organoids from human pluripotent stem cells for modeling myogenesis and muscle regeneration
In vitro organoids derived from human pluripotent stem cells (hPSCs) have been developed as essential tools to study the underlying mechanisms of human development and diseases owing to their structural and physiological similarity to corresponding organs. Despite recent advances, there are a few methodologies for three-dimensional (3D) skeletal muscle differentiation, which focus on the terminal differentiation into myofibers and investigate the potential of modeling neuromuscular disorders and muscular dystrophies. However, these methodologies lacked to recapitulate the developmental processes and lacked regenerative capacity. In this study, we developed a new method to differentiate hPSCs into a 3D human skeletal muscle organoid (hSkMO). This organoid model could recapitulate the myogenesis process and possess regenerative capacities of sustainable satellite cells (SCs), which are adult muscle stem/progenitor cells capable of self-renewal and myogenic differentiation. Our 3D model demonstrated myogenesis through the sequential occurrence of multiple myogenic cell types from SCs to myocytes. Notably, we detected quiescent, non-dividing SCs throughout the hSkMO differentiation in long-term culture. They were activated and differentiated to reconstitute muscle tissue upon damage. Thus, hSkMOs can recapitulate human skeletal muscle development and regeneration and may provide a new model for studying human skeletal muscles and related diseases.
4. Publications
4.1 Journals
4.2 Books and other one-time publications
4.3 Oral and Poster Presentations
1. B. Kuhn (2021-7-6) Imaging neuronal activity with two-photon microscopy in awake mice. Florey Institute of Neuroscience & mental health, Australia. Zoom.
2. B. Kuhn (2021-10-06) Ca2+ activity maps of astrocytes tagged by axo-astrocytic AAV transfer. RIKEN-OIST joint symposium: Kinds of minds - What is thinking? OIST Auditorium and Zoom.
3. B. Kuhn (2022-02-16) The Optical Neuroimaging Unit. OIST Chalk Chat - an introduction for non-researchers. OIST. Zoom.
4. 11th Annual Meeting of Baekryung Medical Symposium, Kangwon National University, Korea. Junghyun Jo: Brain organoids and human disease modeling. September 2021.
5. BK21 Seminar, Korea University Graduate Program for Convergence & Translational Biomedicine, Korea. Junghyun Jo: Midbrain organoids and its applications for studying Parkinson’s disease. September 2021.
6. Korea Brain Research Institute (KBRI) Internal Seminar, Virtual Seminar. Junghyun Jo: Brain organoids for neurodegenerative disease modeling. October 2021.
5. Intellectual Property Rights and Other Specific Achievements
Funding
KAKENHI Scientific Research C
- PI: Junghyun Jo
- Co-Investigator: Gordon Arbuthnott
- Co-Investigator: Zacharie Taoufiq
- Period: 2021 – 2024
JSPS Fellowship
- Mohamed Mostafa Kamal Eltabbal
- Lina Koronfel
- Dr. Melody Lee
6. Meetings and Events
6.1 ICIIBMS 2021
- 6th International Conference on Intelligent Informatics and BioMedical Sciences (ICIIBMS)
- Date: November 25-27, 2021
- Venue: Oita, Kyushu, Japan
- Organized by researchers of the University of the Ryukyus, the Okinawa National College of Technology, and OIST
- 76 participants (20 on site, 56 online) from 8 different countries
6.2 Onna-son/OIST Children’s School of Science 2021
- August 16 - August 20, 2021
- Zoom
- Dr. Kazuo Mori
7. Other
7.1 Planning of ICIIBMS 2022
- The 7th International Conference on Intelligent Informatics and BioMedical Sciences (ICIIBMS) is in the planning phase.
- Date: November 24-26, 2022
- Venue: Nara, Japan
- Organized by researchers of the Okinawa National College of Technology and OIST