Novel Synthesis of 4-Substituted Pyridine-2,6-Dicarboxylic Acid Derivatives (No. 0071)
|
|
|
<< Back to all technologies |
Summary
4-Substituted pyridine-2,6-dicarboxilic acids and their derivatives are important building blocks in the synthesis of bioactive molecules, chemical probes for biological research, solid-supported reagents and other functional molecules. However, known methods for the synthesis of 4-substituted pyridine-2,6-dicarboxylic acids derivatives often require long synthetic routes and harsh conditions. A team of OIST researchers led by Prof. Fujie Tanaka has developed a new synthetic protocol that overcomes the above problems and provides a simple one-pot method to unlock new derivatives.
Applications
- Therapeutic and preventative medicine
- Drug development
- Chemical probes for biological research
Advantages
- Mild conditions
- One-pot reaction
- High atom economy
Technology
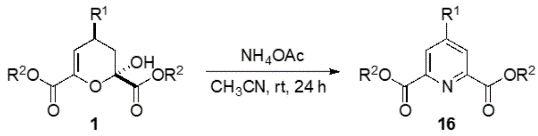
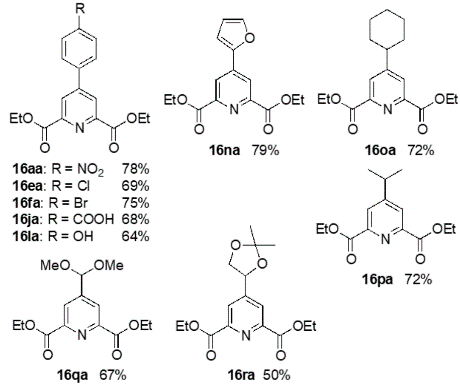
This is a novel method to synthesize chemically and biologically important 4-substituted pyridine-2,6-dicarboxylic acid derivatives, whereby pyruvates and aldehydes are reacted with a pyrrolidine-acetic acid catalyst to form dihydropyran derivatives, which are further reacted with ammonium acetate in one pot under mild conditions to yield the desired products.
Media Coverage and Presentations
CONTACT FOR MORE INFORMATION
![]() Paola Butler-Zanetti
Paola Butler-Zanetti
Technology Licensing Section
![]() tls@oist.jp
tls@oist.jp
![]() +81(0)98-966-8937
+81(0)98-966-8937