One-step diastereoselective synthesis of new sulfonated cyclopropanes and sulfonated linear compounds for drug development (No. 0128)
|
|
|
<< Back to all technologies |
Summary
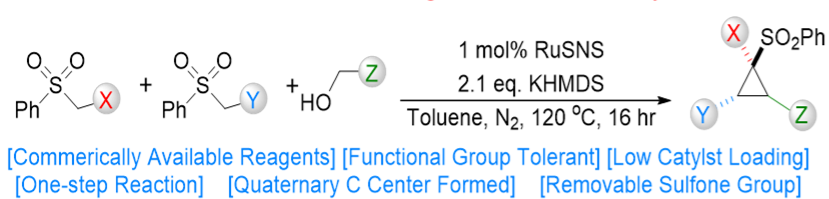
The cyclopropane motif is present in a variety of bioactive products and cyclopropanes are important synthetic precursors in drug development. However, the synthesis of most cyclopropanes requires multiple steps, resulting in lower yields and higher costs. Sulfonated cyclopropanes are particularly difficult to synthesize and their commercial availability is scarce. An OIST research group led by Dr. Eugene Khaskin has developed a rapid and stereoselective synthetic route to produce a wide variety of new cyclopropanes - including sulfone-substituted - in one simple step from low-cost precursors. The technology uses convergent synthesis, where three new carbon-carbon bonds are formed from three components. Convergent synthesis is more attractive than linear, step-up synthesis, since fewer steps and reagents translate into less time and cost. In addition, linear sulfonated equivalents can be obtained through the same reaction simply by tuning the reagents. This novel process unlocks a huge new library of compounds with the potential to be novel synthetic tools for the advancement of medical research and other fields. The OIST team is currently in the process of building such library.
Applications
- Drug design and discovery
- Synthetic precursors
- Protein-interactive moieties
- Lithium-ion batteries
Advantages
- 3-component reaction in one step
- Low-cost precursors
- High diastereoselectivity
Technology
Electron rich, alkyl/aryl, highly substituted cyclopropanes are difficult to synthesize as the current cyclopropanation methods need specialized substrates, often with electron withdrawing groups. Here, three alcohol/ester components are reacted under mild conditions in the presence of a base and a commercially available Ru(II) catalyst. The resulting sulfonated cyclopropanes exhibit good yields and high diastereoselectivity. Alternatively, the equivalent linear sulfonated compounds can be synthesised by tuning the catalyst and stoichiometry of the reagents.
Media Coverage and Presentations
![]() JST Technology Showcase Presentation (EN and JP)
JST Technology Showcase Presentation (EN and JP)
![]() JST Technology Showcase Presentation Slides (JP only)
JST Technology Showcase Presentation Slides (JP only)
CONTACT FOR MORE INFORMATION
![]() Paola Butler-Zanetti
Paola Butler-Zanetti
Technology Licensing Section
![]() tls@oist.jp
tls@oist.jp
![]() +81(0)98-966-8937
+81(0)98-966-8937