'Novel roles of ribosome ubiquitination in quality control systems' Prof. Toshifumi Inada.
Date
Location
Description
Speaker: Prof. Toshifumi Inada
Graduate School of Pharmaceutical Sciences, Tohoku University, Sendai
http://www.pharm.tohoku.ac.jp/~idenshi/inada_lab_HP/Home.html
Title: Novel roles of ribosome ubiquitination in quality control systems
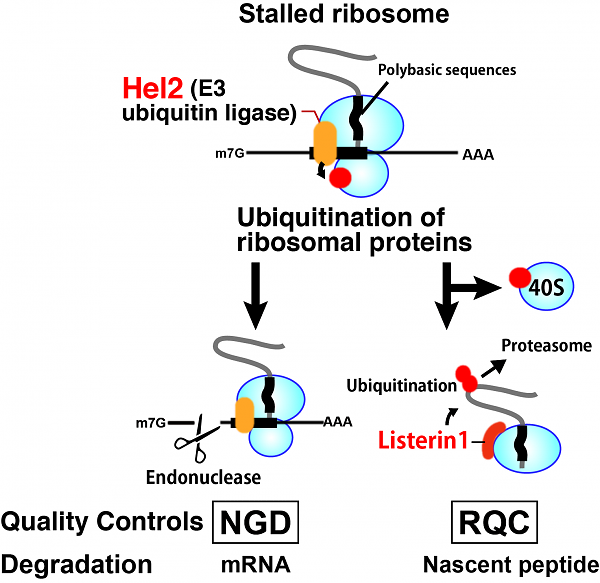
Abstract: Translation elongation rate is regulated to ensure proper conformation and biological function of proteins, and its perturbation induces an endonucleolytic cleavage of the mRNA in the vicinity of the stalled ribosome (NGD: No-Go Decay), and co-translational degradation of the arrested protein product by the proteasome (RQC: Ribosome Quality Control). In RQC, stalled ribosomes are dissociated into subunits, and that an E3 ubiquitin Ltn1 ubiquitylates the peptidyl-tRNA on the 60S subunit, thereby targeting it for proteasomal degradation. Recently we found that an E3 ubiquitin ligase Hel2 (Histone E3 ligase 2) and its ubiquitin-conjugating enzyme Ubc4 are required for NGD and RQC. The ubiquitination of ribosomal proteins by Hel2 is required for both quality control systems. We propose that Hel2 recognizes stalled ribosomes and induces the quality control systems by ubiquitylating specific ribosomal components upon translational arrests.
Attachments
Subscribe to the OIST Calendar: Right-click to download, then open in your calendar application.



