FY2013 Annual Report
Chemistry and Chemical Bioengineering Unit
Professor Dr. Fujie Tanaka, Associate Professor
Abstract
This unit develops (1) efficient, concise, and safe chemical transformation methods and strategies for constructing small molecules bearing functional groups and/or chiral centers, (2) enzyme-like small organic molecule catalysts and protein catalysts to accelerate chemical transformations for the synthesis of functionalized designed molecules, and (3) strategies and tools for the design and selection of highly efficient catalysts and chemical transformations. The research undertaken by this unit advances the chemistry of catalysis and of molecular synthesis. The studies by this unit accelerate the creation of molecules used in biomedical research and contribute to the development of new therapeutics, therapeutic strategies, and diagnostics.
1. Staff
Dr. Hiroyuki Akama, Researcher
Dr. Pandurang V. Chouthaiwale, Researcher
Dr. Hai-Lei Cui, Researcher
Dr. Jithender Enukonda, Researcher
Dr. Andréa N. Forsyth-Norris, Researcher
Dr. Sherida Johnson, Researcher
Dr. Isamu Katsuyama, Researcher
Dr. Hiroshi Nomura, Researcher
Mr. Jyunsuke Machida, Technician
Ms. Motoko Oda, Technician
Mr. Dongxin Zhang, Graduate Student
Mr. Nino Espinas, Rotation Graduate Student
Ms. Nidhi Pant, Research Intern
Ms. Shiho Chinen, Research Administrator
Ms. Tomo Kohatsu, Research Administrator
2. Activities and Findings
2.1. Organocatalysis
We have been developing small organic molecule catalysts (organocatalysts) and organocatalytic molecular transformation methods useful for the synthesis of functionalized molecules under mild conditions in short routes. We are also investigating chemical basis of the catalysis and the chemical transformations to further the creation of useful molecules.
Traditional reaction methods often require high or very low temperature and/or absolute conditions, and many functional groups must be protected. That is, depending on functional groups present in target molecules to be synthesized, synthetic routes including protection and deprotection steps have to be designed for each molecule. To concisely synthesize functionalized molecules, chemical transformation methods that are not affected by functional groups present in starting materials are needed. Therefore, it is a great advantage when a series of molecules bearing various functional groups can be synthesized by the same route without the need of protection and deprotection steps. In addition, it is necessary that such reactions can be performed under safe, mild, and environmentally benign conditions. We address these points in our research to develop catalysts and chemical transformation methods. Our study provides molecules used for actual screening of bioactive candidates and also contributes to create new functional molecules. Our strategies and investigation on the chemical basis of the developed catalysts and methods will further the understanding of the chemistry of organic molecules and their reactions.
2.1.1. Asymmetric hetero-Diels-Alder reactions
One of the recent achievements of this unit is the development of catalytic asymmetric hetero-Diels-Alder reactions that provide functionalized spirooxindole tetrahydropyranones with high diastereo- and enantioselectivities (Scheme 1) (Cui & Tanaka, Chem. Eur. J. 2013, 19, 6213). Novel amine-based catalyst systems have been developed to perform the reactions using enones as reactants. Substituted tetrahydropyranones are important for the syntheses of bioactive molecules. The developed reactions provide concise, atom-economical routes to substituted spirooxindole tetrahydropyranones. The products have the ketone group and the oxindole amide group. Using these groups as further reaction sites, various spirooxindole derivatives were also able to be synthesized. As both the spirooxindole derivatives and the tetrahydropyranes are important as bioactive molecules, the synthesized molecules themselves are of interest. In addition, our reaction methods are useful to provide a wide range of functionalized spirooxindole tetrahydropyrans to synthesize bioactive candidates and their synthetic intermediates.
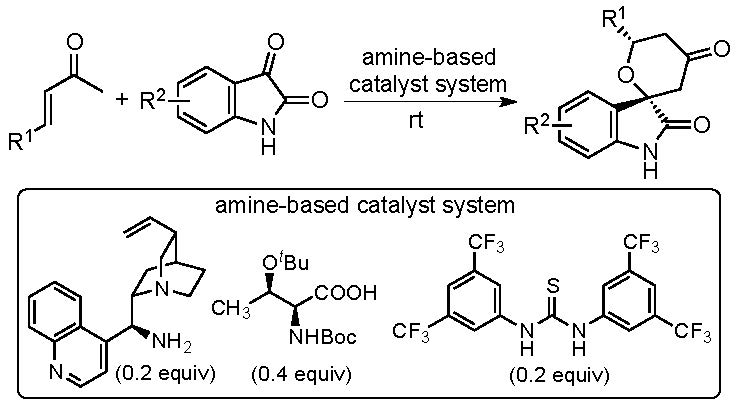
Scheme 1
Because the hetero-Diels-Alder reactions that directly use enones as starting materials without the requirement of pre-formation of dienes for the hetero-Diels-Alder reactions are significant, we have been investigating the mechanism of the reactions and key factors for the high diastero- and enantioselectivities.
We have also been studying to expand the oxa-Diels-Alder reactions to those that use various ketones and aldehydes as dienophiles beyond the use of the oxindole dienophiles, and to aza-Diels-Alder reactions that use imines as dienophile reactants.
2.1.2. Synthesis of furanose spirooxindoles via fast aldol reactions
Spirooxindoles are important as they are often found in bioactive molecules as described in the section of the hetero-Diels-Alder reactions. Because furanose units are present in biomolecules, furanose spirooxindoles may be useful as biofunctional molecule candidates. We have designed and synthesized furanose spirooxindoles (Scheme 2) (Zhang, Johnson, Cui & Tanaka, Asian J. Org. Chem. 2014, 3, 391). In our design, the first step was an aldol reaction of a pyruvic aldehyde derivative 1,1-dimethoxypropan-2-one with an isatin; the following reduction of the ketone carbonyl group generated the furanose spiro system.
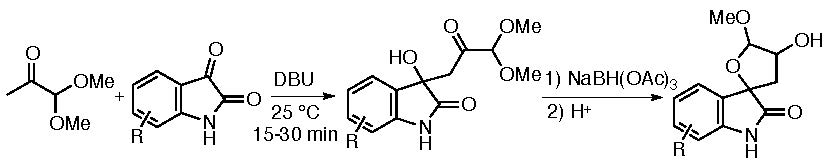
Scheme 2
2.1.3. One-pot synthesis of polysubstituted 3-acylpyrroles
Substituted 3-acylpyrroles are present in many pharmaceuticals and biologically active molecules. We have developed a concise method to synthesize 3-acylpyrroles in one pot from readily available unsaturated ketones and N-substituted propargylated amines via aza-Michael-alkyne carbocyclization cascade followed by oxidation (Scheme 3) (Cui & Tanaka, Org. Biomol. Chem. 2014, 12, 5822).
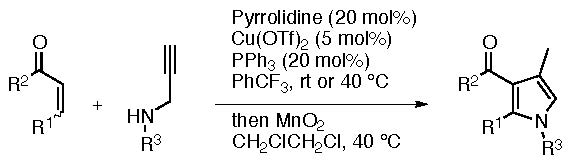
Scheme 3
2.2. Fluorescence-based reaction monitoring systems
This unit has also been developing concise fluorescence-based assay methods to monitor bond-forming and bond-breaking reaction progress on a small scale to facilitate the development of catalysts and catalyzed chemical transformation methods. Use of fluorogenic substrates provides a straightforward method of reaction monitoring because reaction progress is directly observed as an increase in fluorescence.
We have developed fluorogenic aldehydes based on a diarylacetylene core structure and the fluorescence-based assay systems for aldol reactions using the fluorogenic aldehydes (Scheme 4) (Katsuyama, Chouthaiwale, Akama, Cui & Tanaka, Tetrahedron Lett. 2014, 55, 74). We have previously found that electronic features of substituents are significantly less influential in a diphenylacetylene system than in a simple benzene system (Katsuyama, Chouthaiwale, Cui, Ito, Sando, Tokiwa & Tanaka, Tetrahedron 2013, 69, 4098). That is, when diarylacetylene derivatives are used as fluorogenic substrate core structures, design of functions of the molecules can be the main focus, as reactivity does not depend on substituent. By taking advantage of the feature of diarylacetylene structure, useful fluorogenic assay systems were created.
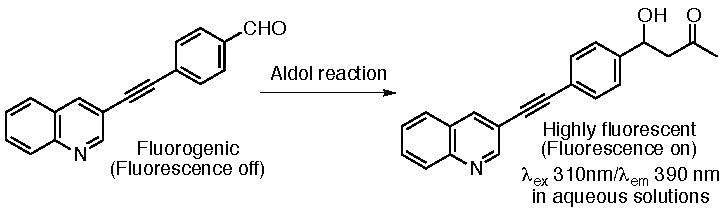
Scheme 4
3. Publications
3.1 Journals
- Cui, H.-L. & Tanaka, F. Catalytic enantioselective formal hetero-Diels-Alder reactions of enones with isatins to give spirooxindole tetrahydropyranones. Chemistry - A European Journal 19, 6213-6216 (2013), doi: 10.1002/chem.201300595. Corrigendum: Chemistry - A European Journal 19, 12187 (2013).
- Katsuyama, I., Chouthaiwale, P. V., Cui, H.-L., Ito, Y., Sando, A., Tokiwa, H. & Tanaka, F. Substituent-dependent reactivity in aldehyde transformations: 4-(phenylethynyl)benzaldehydes versus simple benzaldehydes. Tetrahedron 69, 4098-4104 (2013), doi: 10.1016/j.tet.2013.03.056.
- Mase, N., Takabe, K. & Tanaka, F. Fluorogenic probes for chemical transformations: 9-anthracene derivatives for monitoring reaction progress by an increase in fluorescence. Tetrahedron Letters 54, 4306-4308 (2013), doi: 10.1016/j.tetlet.2013.06.010. Erratum: Tetrahedron Letters 54, 5140 (2013).
- Katsuyama, I., Chouthaiwale, P. V., Akama, H., Cui, H.-L. & Tanaka, F. Fluorogenic probes for aldol reactions: Tuning of fluorescence using p-conjugation systems. Tetrahedron Letters 55, 74-78 (2014), doi: 10.1016/j.tetlet.2013.10.122.
- Mase, N., Ando, T., Shibagaki, F., Sugita, A., Narumi, T., Toda, M., Watanabe, N. & Tanaka, F. Fluorogenic aldehydes bearing arylethynyl groups: turn-on aldol reaction sensors for evaluation of organocatalysis in DMSO. Tetrahedron Letters 55, 1946-1948 (2014), doi: 10.1016/j.tetlet.2014.02.007.
- Zhang, D., Johnson, S., Cui, H.-L. & Tanaka, F. Synthesis of furanose spirooxindoles via 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU)-catalyzed aldol reactions of a pyruvic aldehyde derivative. Asian Journal of Organic Chemistry 3, 391-394 (2014), doi: 10.1002/ajoc.201400016.
- Cui, H.-L. & Tanaka, F. One-pot synthesis of polysubstituted 3-acylpyrroles by cooperative catalysis. Organic & Biomolecular Chemistry 12, 5822-5826 (2014), doi: 10.1039/c4ob01019a.
3.2. Other Publications
- Tanaka, F. Synthesis of functionalized molecules and catalyst development strategies (官能基化された分子の合成と触媒開発戦略). In MEXT Grant Advanced Molecular Transformations by Organocatalysts News Letter (文部科学省科学研究費補助金「新学術領域研究」有機分子触媒による未来型分子変換 News Letter) No. 21 (2013)
3.3. Oral and Poster Presentations
- Chouthaiwale, P. V. & Tanaka, F. Amino acid-catalyzed reactions of pyruvates: One-pot aldol condensation-Michael addition-cyclization sequence, in Advanced Molecular Transformations by Organocatalysts 1st International Conference & 6th Symposium on organocatalysis, Otsu, Japan (2013), 2013.05.27-2013.05.28.
- Cui, H.-L. & Tanaka, F. Catalytic enantioselective formal hetero-Diels-Alder reactions of enoneswith isatins to give spirooxindole tetrahydropyranones, in Advanced Molecular Transformations by Organocatalysts 1st International Conference & 6th Symposium on Organocatalysis, Otsu, Japan (2013), 2013.05.27-2013.05.28.
- Tanaka, F. Synthesis of sugar derivatives for screening of bioactive molecules, in The 1st Committee Meeting (H25), Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”, Naha, Okinawa, Japan (2013), 2013.06.20.
- Tanaka, F. Synthesis of sugar derivatives and sugar-related molecules for the search of bioactive molecules, in Symposium, Okinawa Intellectual Cluster Program, Naha, Okinawa, Japan (2013), 2013.12.19.
- Tanaka, F. Synthesis of sugar derivatives for screening of bioactive molecules, in The 2nd Committee Meeting (H25), Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”, Naha, Okinawa, Japan (2013), 2014.02.10.
- Cui, H.-L. & Tanaka, F. Cooperative catalysis: synthesis of 3-acylpyrroles and 2,5-dihydropyrroles via aza-Michael-alkyne carbocyclization cascade from enones and propargylamines, in The 134th Annual Meeting of the Pharmaceutical Society of Japan (2014), Kumamoto, Japan (2014), 2014.03.27-2014.03.30.
- Johnson, S. & Tanaka, F. Organocatalytic aldol reactions of C6 pyranoses to synthesize C9 sugars, in The 134th Annual Meeting of the Pharmaceutical Society of Japan (2014), Kumamoto, Japan, 2014.03.27-2014.03.30.
- Zhang, D., Johnson, S., Cui, H.-L. & Tanaka, F. Synthesis of spirooxindole furanose derivatives via DBU-catalyzed aldol reactions of pyruvic aldehyde dimethyl acetal, in The 134th Annual Meeting of the Pharmaceutical Society of Japan (2014), Kumamoto, Japan, 2014.03.27-2014.03.30.
- Cui, H.-L. & Tanaka, F. Asymmetric hetero-Diels Alder reaction: Synthesis of spirooxindole-bearing tetrahydropyranones, in Screening Committee of Anticancer Drugs 3rd Symposium, Natural Products in the Academia-originated Development of Anticancer Drugs (文科省新学術領域研究•がん支援「化学療法基盤支援活動」第3回シンポジウム アカデミアからの抗がん剤創薬に向けて天然物の有効利用), Nago, Okinawa, Japan (2014), 2014.05.12.
- Tanaka, F. Synthesis of sugar derivatives for screening of bioactive molecules, in The 1nd Committee Meeting (H26), Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”, Naha, Okinawa, Japan (2014), 2014.06.09.
- Cui, H.-L. & Tanaka, F. Asymmetric formal oxa-Diels Alder reaction catalyzed by amine-based catalysts affording functionalized spirooxindole tetrahydropyrans, in The 14th Belgian Organic Synthesis Symposium, Louvain-la-Neuve, Belgium (2014), 2014.07.13-2014.07.18.
- Zhang, D. Johnson, S., Cui, H.-L. & Tanaka, F. Synthesis of Spirooxindole Furanose Derivatives via DBU-Catalyzed Aldol Reactions, in The 14th Belgian Organic Synthesis Symposium, Louvain-la-Neuve, Belgium (2014), 2014.07.13-2014.07.18.
4. Intellectual Property Rights (Patent Applications)
- Cui, H.-L. & Tanaka, F. Novel spirooxindole derivative and process for producing the same. PCT/JP2013/077682 (2013). (application date: 2013.10.04)
- Chouthaiwale, P. V. & Tanaka, F. Process for producing dihydro-2H-pyran derivatives. PCT/JP2013/078249 (2013). (application date: 2013.10.10)
5. External Funding
- Grant-in-Aid for Scientific Research on Innovative Areas “Advanced Molecular Transformations by Organocatalysts” from The Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan
Title: Enamine-based organocatalytic molecular transformations: Development of highly efficient catalysts
PI: Tanaka, Fujie
April 2013 – March 2014
- Grant-in-Aid for Scientific Research on Innovative Areas “Advanced Molecular Transformations by Organocatalysts” from The Ministry of Education, Culture, Sports, Science and Technology (MEXT); Japan Society for the Promotion of Science (JSPS), Japan
Title: Development of efficient amine-based catalyst systems
PI: Tanaka, Fujie
April 2014 – March 2015
- Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”
Title: Synthesis of sugar derivatives for screening of bioactive molecules
PI: Tanaka, Fujie
April 2013 – March 2014
- Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”
Title: Synthesis of sugar derivatives for screening of bioactive molecules
PI: Tanaka, Fujie
April 2014 – March 2015
6. Meetings and Events
6.1 Seminars
- Date: February 14, 2014
- Venue: OIST campus
- Speaker: Professor Takahiko Akiyama, Department of Chemistry, Gakushuin University, Japan
- Two (2) seminars:
- Title 1: Development of chiral phosphoric acid and its application to enantioselective reactions
- Title 2: Recent progress in the chiral phosphoric acid catalysis
6.2 Seminar
- Date: February 19, 2014
- Venue: OIST campus
- Speaker: Professor Jun’ichi Kobayashi, Graduate School of Pharmaceutical Sciences, Hokkaido University, Japan
- Title: Bioactive natural products from Okinawan marine organisms
6.3 Seminar
- Date: August 21, 2014
- Venue: OIST campus
- Speaker: Professor Keiji Maruoka, Department of Chemistry, Graduate School of Science, Kyoto University, Japan
- Title: Challenges in Organocatalytic Chemistry: Design of Organoradical Catalysts and their Synthetic Application



